Cell and Molecular Immunlogy1-Cells and Tissues of Immune System
History:
Immunology
is a medical science that examines the structure and function of the immune
system. The earliest known noted information to immunity was during the plague in Athens
in 430 BC. They did
notice that people who had recovered from a previous bout of the disease could
nurse the sick without contracting the illness a second time. In the 18th
century, Pierre-Louis Moreau de Maupertuis
made experiments with scorpion venom and observed that certain dogs and mice
were immune to this venom. This and other observations of acquired immunity
were later provided insight to Louis Pasteur
in his efforts to develop vaccination
and he proposed germ theory of disease.
Pasteur's theory was in direct opposition to contemporary theories of disease,
such as the miasma theory.
�It was not until Robert Koch
in 1891 provided proofs,
for which he was awarded a Nobel Prize
in 1905, that microorganisms
were confirmed as the cause of infectious disease.
With the discovery of the yellow fever
viruses by Walter Reed
viruses were confirmed as human pathogens in 1901 (Wiki
P).
Towards the end of the 19th century, study of humeral immunity and cellular immunity has revolutionized the field of Immunology. Particularly, Paul Ehrlich�s work was important for he proposed the side-chain theory to explain the specificity of the antigen-antibody reaction; his contributions in the understanding of humeral immunity was recognized and awarded of Nobel Prize to him in 1908, which was jointly awarded to the founder of cellular immunology,- ElieHYPERLINK "http://en.wikipedia.org/wiki/Elie_Metchnikoff" Metchnikoff.
Immunity against microorganisms in our body is basically two stage processes; one called innate immunity and another adaptive immunity. �Innate immunity initiates within few hours of infection, which has no memory but adaptive immunity is triggered by innate immunity, and takes some time and acquires memory.� The cells and the systems involved in these processes are different.
Myeloid cells in innate and adaptive immunity:
https://www.cancer.gov;https://www.slideshare.net/
http://www.ncbi.nlm.nih.gov/
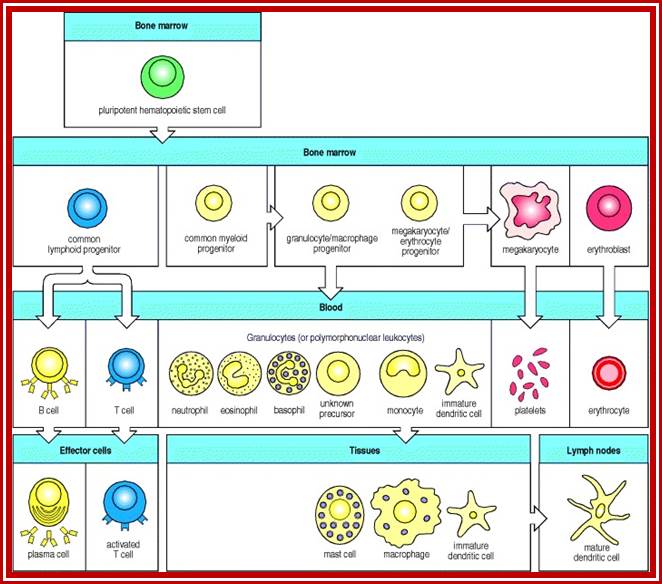
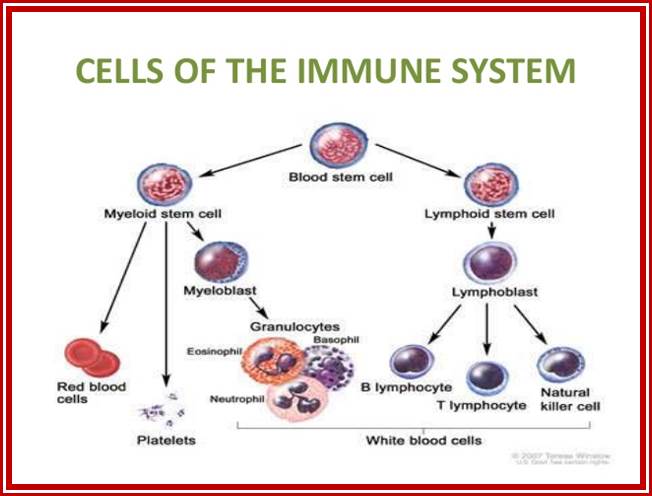
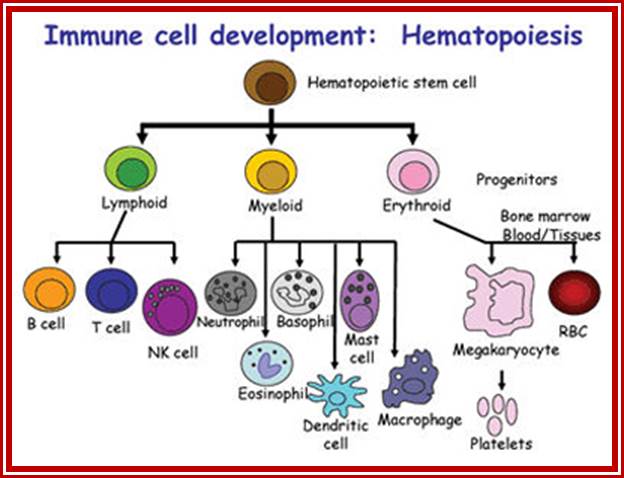
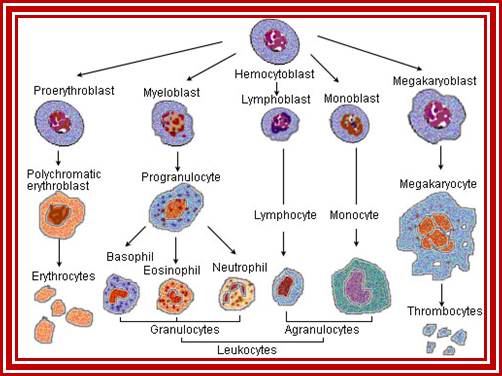
All the cellular elements of blood, including the lymphocytes of the adaptive immune system, arise from hematopoietic stem cells in the bone marrow; https://www.urmc.rochester.edu
These pluripotent cells divide to produce two more specialized types of stem cells, a common lymphoid progenitor that gives rise to the T and B lymphocytes responsible for adaptive immunity, and a common myeloid progenitor that gives rise to different types of leukocytes (white blood cells), erythrocytes (red blood cells that carry oxygen), and the megakaryocytes that produce platelets that are important in blood clotting. The existence of a common lymphoid progenitor for T and B lymphocytes is strongly supported by current data. T and B lymphocytes are distinguished by their sites of differentiation-T cells in the thymus and B cells in the bone marrow�and by their antigen receptors. �Mature T and B lymphocytes circulate between the blood and peripheral lymphoid tissues. After they encounter with antigen, B cells differentiate into antibody-secreting plasma cells, whereas T cells differentiate into effector T cells with a variety of functions. A third lineage of lymphoid-like cells, the natural killer cells, derive from the same progenitor cell but lack the antigen-specificity that is the hallmark of the adaptive immune response (not shown). The leukocytes that derive from the myeloid stem cell are the monocytes, the dendritic cells, and the basophils, eosinophils and neutrophils. The latter three are collectively termed both granulocytes, because of the cytoplasmic granules whose characteristic staining gives them a distinctive appearance in blood smears, or polymorphonuclear leukocytes, because of their irregularly shaped nuclei. They circulate in the blood and enter the tissues only when recruited to sites of infection or inflammation where neutrophils are recruited to phagocytose bacteria. Eosinophils and basophils are recruited to sites of allergic inflammation, and appear to be involved in defending against parasites. Immature dendritic cells travel via the blood to enter peripheral tissues, where they ingest antigens. When they encounter a pathogen, they mature and migrate to lymphoid tissues, where they activate antigen-specific T lymphocytes.Monocytes enter tissues, where they differentiate into macrophages; these are the main tissue-resident phagocytic cells of the innate immune system. Most cells arise from precursors in bone marrow but complete their maturation in tissues; they are important in allergic responses. http://www.ncbi.nlm.nih.gov/
http://anuvrat.info/
http://fourthingsabout.blogspot.com
Myeloid cells in innate and adaptive immunity:
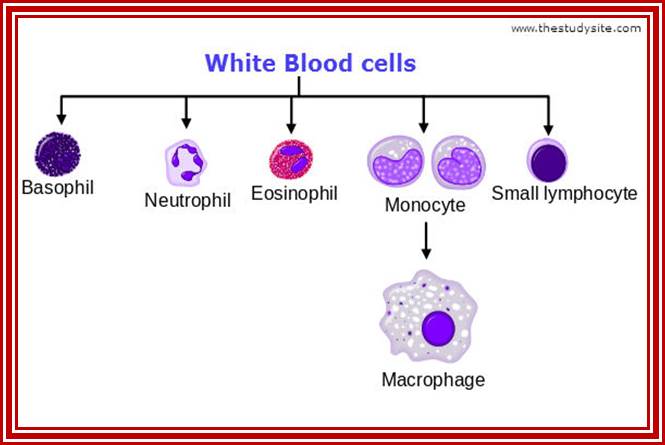
Cells of the myeloid lineage perform various important functions in the immune response. The cells are shown schematically in the left column in the form in which they will be represented throughout the rest of the book. A photomicrograph of each cell type is shown in the center column. Macro-phages and neutrophils are primarily phagocytic cells that engulf pathogens and destroy them in intracellular vesicles, a function they perform in both innate and adaptive immune responses. Dendritic cells are phagocytic when they are immature and take up pathogens; after maturing they act as antigen-presenting cells to T cells, initiating adaptive immune responses. Macrophages can also present antigens to T cells and can activate them. The other myeloid cells are primarily secretory cells that release the contents of their prominent granules upon activation via antibody during an adaptive immune response. Eosinophils are thought to be involved in attacking large antibody-coated parasites such as worms, whereas the function of basophils is less clear. Mast cells are tissue cells that trigger a local inflammatory response to antigen by releasing substances that act on local blood vessels. http://www.ncbi.nlm.nih.gov/
|
Components of the immune system |
|
|
|
|
|
Response is non-specific |
Pathogen and antigen specific response |
|
Cell-mediated and humoral components |
Cell-mediated (T cell) and humoral components (B cell) |
|
Exposure leads to immunological memory |
|
|
Found in nearly all forms of life |
Found only in jawed vertebrates |
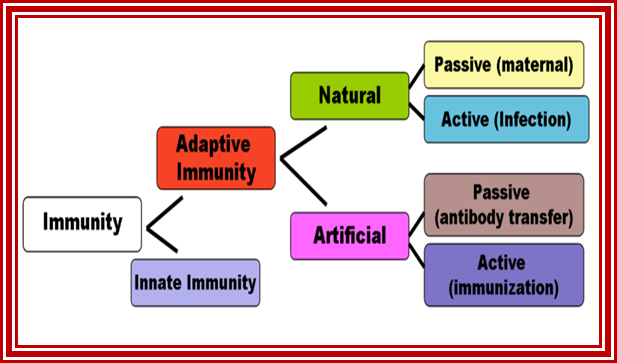
Flow chart diagram depicting the divisions of Immunity Natural immunity occurs through contact with a disease-causing agent, when the contact was not deliberate, whereas artificial immunity develops only through deliberate actions of exposure. Both natural and artificial immunity can be further subdivided, depending on the amount of time the protection lasts. Passive immunity is short lived, and usually lasts only a few months, whereas protection via active immunity lasts much longer, and is sometimes life-long.; WIKIPEDIA medical: https://en.wikipedia.org
Animal Cells and Tissues of Immune system;
1. Animal Cell Structure:
Understanding of animal cell structure is fundamental for understanding the animal system. Eukaryotic animal cells are enclosed in a plasma membrane and inside one finds membrane-bound nucleus and cell organelles all membrane bound. Unlike the eukaryotic cells of plant cells do not have a cell wall. This feature was lost in the distant past by the single-celled organisms that gave rise to the kingdom Animalia. Most cells, both animal and plant, range in size between 1 and 100 micrometers and are thus visible only with the aid of a microscope.
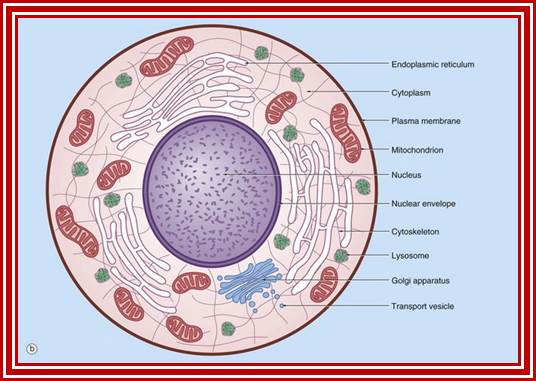
Schematic diagram;
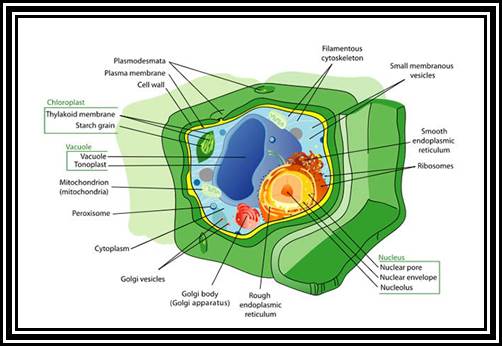
The cell (illustration opposite) Diagram.
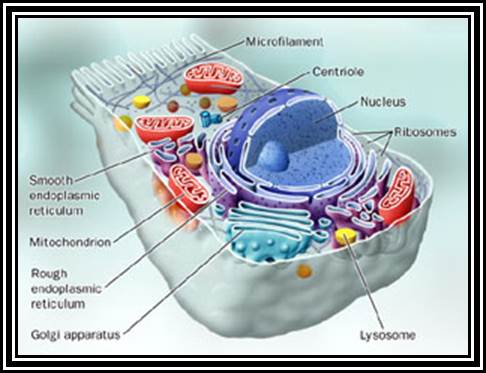
ULTRASTRUCTURE OF THE EUKARYOTIC CELLs (Diagram):
ULTRASTRUCTURE OF THE EUKARYOTIC CELLs: The cell is the living functional unit of all organisms. An organism may be composed of one cell only (Unicellular) e.g., Bacteria and Algae or of several cells (Multicellular) e.g. Man. The cell exists in two forms: 1. Eukaryotic cell, which has a nucleus that is enclosed in a nuclear envelope and several membranes- limited compartments e.g., the human cell. 2. Prokaryotic cell which has no nucleus and is devoid of membrane-limited compartments e.g. the bacterial cell. Structure of the Eukaryotic Cell (See Fig. C1) A broad spectrum of morphological and functional specializations of cells occurs in the multicellular organisms. However, all Eukaryotic cells conform to a basic structural model. Thus, the eukaryotic cell is composed of two basic parts; the Cytoplasm and the Nucleus. THE NUCLEUS This is the largest organelle of the cell often located in the central part of the cytoplasm and enclosed in a double-layered nuclear membrane. Its shape usually corresponds to the shape of the cell in which it is found. It contains a nucleolus/nucleolus (which produces ribosomal subunits) and chromatin (DNA). The latter is the genetic material implicated in cell division and in the synthesis of several molecules particularly proteins. The nucleus as the control centre of the cell contains the blueprint for all cellular structure and activities and in its absence the cell can neither function nor survive. The nuclear envelope is perforated by pores (3000-4000), which facilitate communication between the nucleus and the cytoplasm. Attached to the outer membrane of the nuclear envelope are polyribosomes. This membrane is also continuous with the rough endoplasmic reticulum of the cytoplasm. THE CYTOPLASM. The fluid component of the cytoplasm is the Cytosol (pH 7.2) while the metabolically active contents of the cytoplasm are the Organelles. Apart from being metabolically active, organelles are permanent residents of the cell which would survive cell division i.e., they reappear in the daughter cells following cell division. Organelles occur in two forms, freely within the cytosol or enclosed in membrane. The cytoplasm also contains substances which are not metabolically active. These are called Inclusion Bodies. Inclusion bodies are the transitory residents of the cytoplasm not involved in cell metabolism and do not survive cell division. They comprise mainly accumulated metabolites, lipid droplets, pigments and minerals and are sporadically distributed in body cells. The eukaryotic cell is enclosed in a limiting membrane called the Plasma membrane or the Plasmalemma. 2 Fig C1: Ultrastructure of the cell THE PLASMALEMMA Fig. C 2 The Plasmalemma is the physical external boundary of the cell.
Ultrastructure of the cell THE PLASMALEMMA Fig. C 2 The Plasmalemma is the physical external boundary of the cell. It is composed of Phospholipids, Protein, Carbohydrate and Cholesterol which are intricately organised into a trilaminar structure. The cell membrane as well as the membranes surrounding the organelles ranges from 7.5 to 10 nanometre in thickness. The 3- layered structure, referred to as the Unit Membrane structure is formed from two phospholipid layers; the fatty acid nonpolar (Hydrophobic) tails of the two phospholipids are located in the middle layer while the polar (Hydrophilic) head are located on either side of the middle layer (see Fig. C2). The proteins of the cell membrane account for 50% (w/w) of the membrane and occur in two forms, viz. integral proteins which traverse the thickness of the membrane and peripheral proteins which are adsorbed to the outer or inner surfaces of the membrane. While the peripheral proteins are involved in cell recognition and interactions, integral proteins regulate passage of material and active transport of specific molecules across the membrane.
The basic structural features, common to all eukaryotic cells, are illustrated in this graphic picture (a) a fibroblast and diagram (b). All cells are bounded by an external lipid membrane, called the plasma membrane or plasmalemma PM, which serves as a dynamic interface with the external environment. Most cells interact with two types of external environment: adjacent cells C and extracellular matrix as represented by collagen fibrils F. The space between cells is designated the intercellular space IS. The functions of the plasma membrane include transfer of nutrients and metabolites, attachment of the cell to adjacent cells and extracellular matrix, and communication with the external environment.� The nucleus N is the largest organelle and its substance is bounded by a membrane system called the nuclear envelope or membrane NE. The nucleus contains the genetic material of the cell in the form of Deoxyribose Nucleic Acid (DNA). The cytoplasm contains a variety of other organelles, many of which are also bounded by membranes. An extensive system of flattened membrane-bound tubules, saccules and flattened cisternae, collectively known as the endoplasmic reticulum ER, is widely distributed throughout the cytoplasm. A second discrete system of membrane-bound saccules, the Golgi apparatus G, is typically located close to the nucleus (best seen in the adjacent cell). Scattered free in the cytoplasm are a number of relatively large, elongated organelles called mitochondria M, which have a smooth outer membrane and a convoluted inner membrane system. In addition to these major organelles, the cell contains a variety of other membrane-bound structures, including intracellular transport vesicles V and lysosomes L. The cytoplasmic organelles are suspended in a gel-like medium called the cytosol, in which many metabolic reactions take place. Within the cytosol, there is a network of minute tubules and filaments, collectively known as the cytoskeleton, which provides structural support for the cell and its organelles, as well as providing a mechanism for transfer of materials within the cell and movement of the cell itself.� Thus, the cell is divided into a number of membrane-bound compartments, each of which has its own particular biochemical environment. �Membranes, therefore serve to separate incompatible biochemical and physiological processes. In addition, enzyme systems are found anchored in membranes so that membranes are themselves the site of many specific biochemical reactions. Membrane-enclosed compartments occupy approximately half the volume of the cell. C adjacent cell, ER endoplasmic reticulum, F collagen fibrils, G Golgi apparatus, IS intercellular space, L lysosome, M mitochondrion, N nucleus, NE nuclear envelope, PM plasma membrane, V transport vesicles. https://basicmedicalkey.com/
TEM picture of the Nucleus:
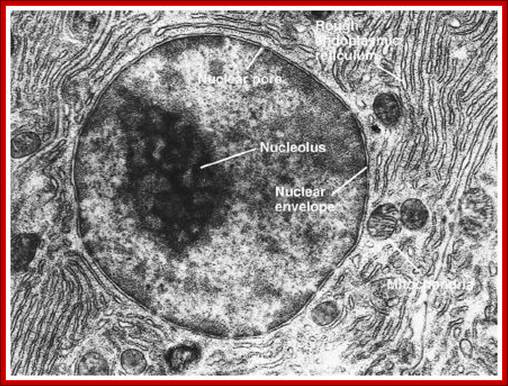
http://www.scienceclarified.com/images/uesc_03_img0119.jpg
Cells once get differentiated into different cell types, such differentiated cell types with specific structure and function in the human body can be any- where 325 types among 10^14 to-10^16 cells that go into human body formation. There will lot turnover over of cells, but not brain cells.
|
Structure |
Composition |
Function/s |
|
Cell wall* Plants |
Contains cellulose |
Support and protection |
|
Plasma membrane |
Phospholipids and proteins |
Boundary. Transport and receptors |
|
Nucleus |
Envelope, chromatin, nucleoli |
Genetic information |
|
Nucleolus |
rRNA synthesis |
Ribosome units |
|
Ribosomes |
Riboproteins and RNA |
Protein synthesis |
|
Endoplasmic Reticulum |
Membranes-sheets |
Protein synthesis and transport of proteins |
|
RER Rough ER |
Ribosomes bound |
Protein synthesis |
|
SER, smooth ER |
Free from ribosomes |
Protein transport to destinations |
|
Golgi bodies |
Stack of membranes |
�Protein processing and packaging and delivery |
|
Lysosomes |
membrane vesicles,- |
digestive function |
|
Vacuoles and vesicles |
Membranous sacs storage |
|
|
Peroxisomes |
Membranous vesicles |
Peroxidase function |
|
Mitochondria |
Membrane-inner and outer |
ATP production |
|
Chloroplasts-plants |
Membrane bound |
photosynthesis |
|
Cytoskeleton MTs Actins, Intermediate filaments |
Mechanical |
|
|
Celia and flagella |
9+2-MTsProteins |
mobility |
|
Centriole |
9+0 pattern MTs |
Form basal bodies |
|
Extra cellular matrix |
Surround the PM -collagen |
Bring cells together |
|
Glyoxysomes-plants |
Enzymes for fatty acid metabolism |
|
The lack of a rigid cell wall allowed animals to develop a greater diversity of cell types, tissues, and organs. Specialized cells that formed are nerves and muscle, tissues impossible for plants to evolve, gave these organisms mobility. Lower plants such as algae are mobile. The ability to move about by the use of specialized muscle tissues and flagella and Celia is a hallmark of the animal world, though a few animals, primarily sponges, do not possess differentiated tissues.� Notably, protozoan move, but only via nonmuscular means, in effect, use cilia, flagella, and pseudopodia.
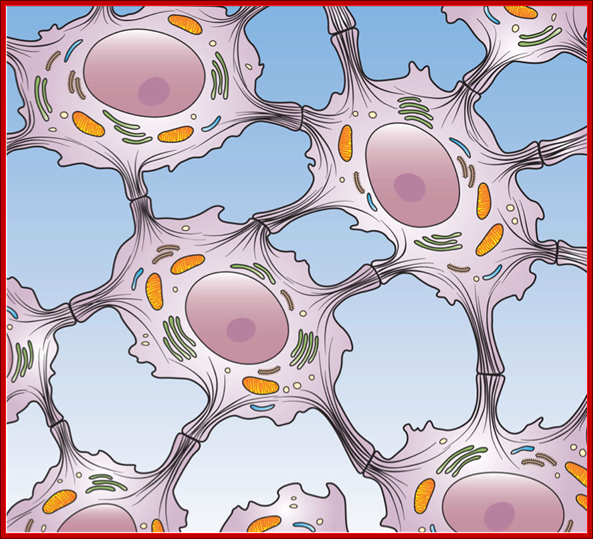
The animal kingdom is unique among eukaryotic organisms because most animal tissues are bound together in an extracellular matrix by a triple helix of protein known as collagen. Plant and fungal cells are bound together in tissues or aggregations by other molecules, such as pectin and hemicelluloses. The fact that no other organisms utilize collagen in this manner is one of the indications that all animals arose from a common unicellular ancestor. Bones, shells, spicules, and other hardened structures are formed when the collagen-containing extracellular matrix between animal cells becomes calcified.
Animals are a large and incredibly diverse group of organisms. Making up about three-quarters of the species on Earth, they run the gamut from corals and jellyfish to ants, whales, elephants, and, of course, humans. Being mobile, Amoeba n=27 animals, which are capable of sensing and responding to their environment, with flexibility they adopt different modes of feeding, defence, and reproduction. Unlike plants, however, animals are unable to manufacture their own food except some chloroplast containing euglena like species, and therefore, animals are always directly or indirectly dependent on plant life.
Most animal cells are diploid, but amoeba forms haploids (n=27); meaning that their chromosomes exist in homologous pairs. Different chromosomal (ploids) are also, however, known to occasionally occur mostly in plant systems. The proliferation of animal cells occurs in a variety of ways, asexual by mitosis or by spore formation.� Many perform sexual reproduction, the cellular process of meiosis is first necessary so that haploid daughter cells or gametes, can be produced. Two haploid cells then fuse to form a diploid zygote, which develops and grows by cell division and differentiation.
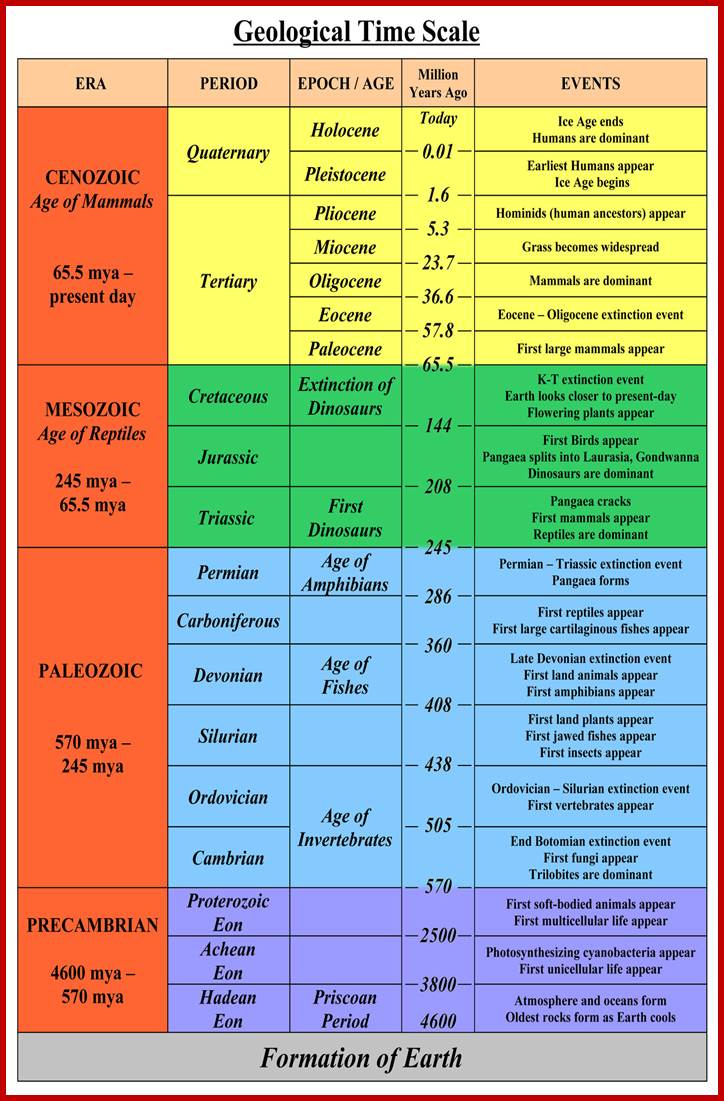
JamesvilleMiddleScience-wikispace.com2002
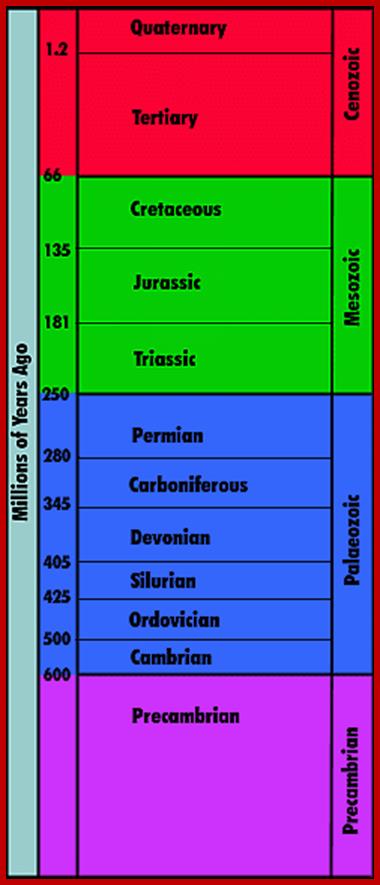
Time scale; http://earthsci.org/fossils/youngp/periods/periods.html
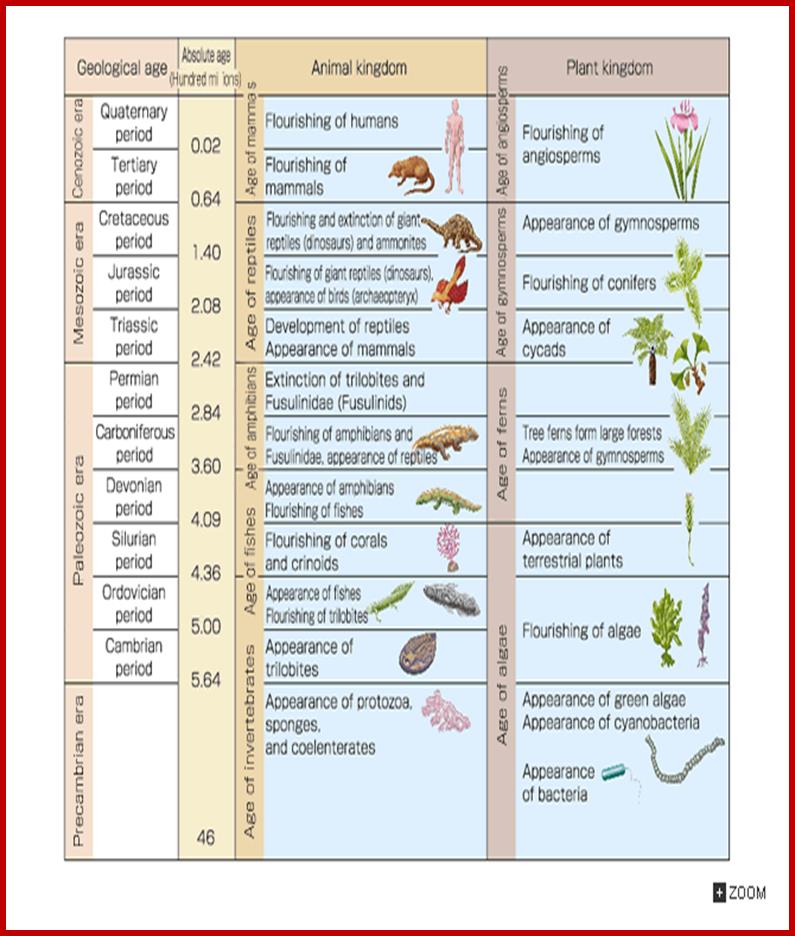
Geological Time Scale Divisions and the Emergence of Life; http://csls-text2.c.u-tokyo.ac.jp/inactive/01_03.html
The earliest fossil evidence of animal�s dates from the Vendian Period (650 to 544 million years ago), with coelenterate-type creatures that left traces of their soft bodies in shallow-water sediments. The first mass extinction ended in that period, but during the Cambrian Period which followed, an explosion of new forms began the evolutionary radiation that produced major groups or phyla, known today. Vertebrates (animals with backbones) are not known to have occurred until the early Ordovician Period (505 to 438 million years ago).
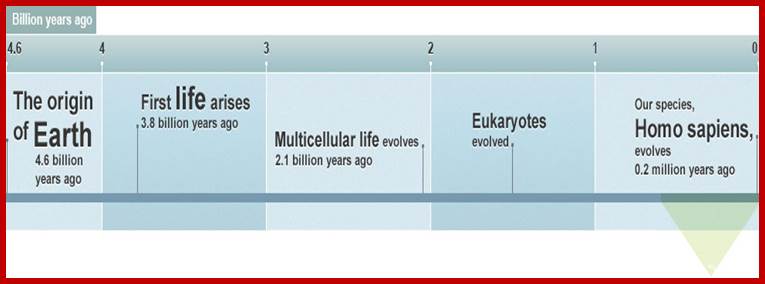
http://www.bbc.co.uk/nature/history_of_the_earth
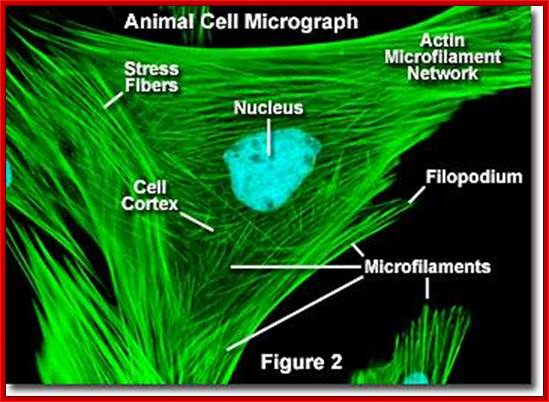
Molecular expressions- microfilament; http://micro.magnet.fsu.edu/
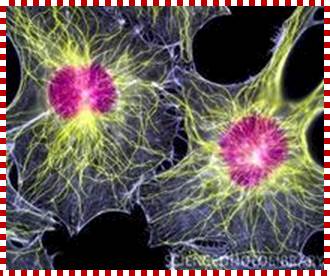
Cells were discovered in 1665 by British scientist Robert Hooke who first observed them by his crude (by today's standards) seventeenth century optical microscope. In fact, Hooke coined the term "Cell", in a biological context, when he described the microscopic structure of cork like, a tiny, bare monk's cell (enclosed compartment to live). Illustrated in the above figure are pair of fibroblast deer skin cells that have been labelled with fluorescent probes and photographed in the microscope to reveal their internal structure. The nuclei are stained with a bluish probe, while the Golgi apparatus and microfilament actin network are stained green and blue, respectively. The microscope has been a fundamental tool in the field of cell biology and is often used to observe living cells in culture.
This image shows a desmosome junction between cells of the epidermal layer of the skin: http://en.wikipedia.org/
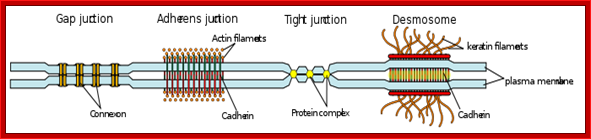
Examples of cell junctions; http://en.wikipedia.org/
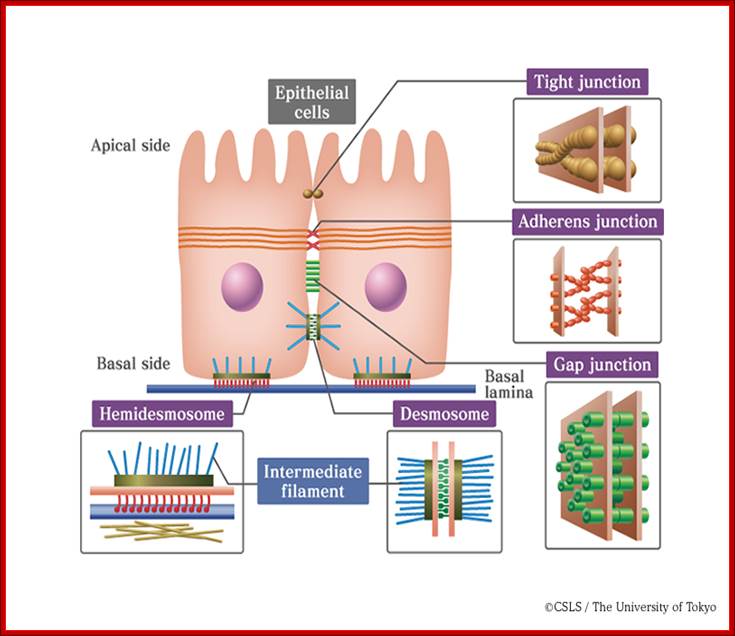
Extracellular space components; Epithelial cells are connected by adhesion structures called junctional complexes, which consist of tight junctions, desmosomes and gap junctions on the lateral side. �������������� On the basal side, cells are adhered to basal laminae by hemidesmosomes. Junctional complexes that connect epithelial cells;���� http://csls-text.c.u-tokyo.ac.jp/large_fig/
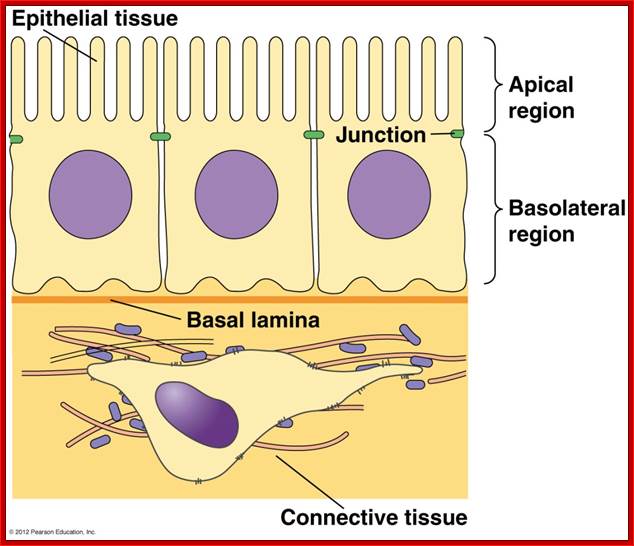
Principles of Biology: http://www.mun.ca/
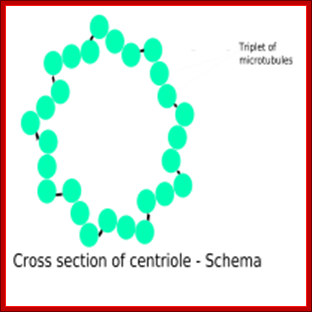
Centrioles - Centrioles are self-replicating organelles made up of nine microtubule bundles and they are found only in animal cells. They appear to help in organizing cell division, but aren't essential to the process. Edouard van Beneden and Theodor Boveri (1883-1888) made the first observation.
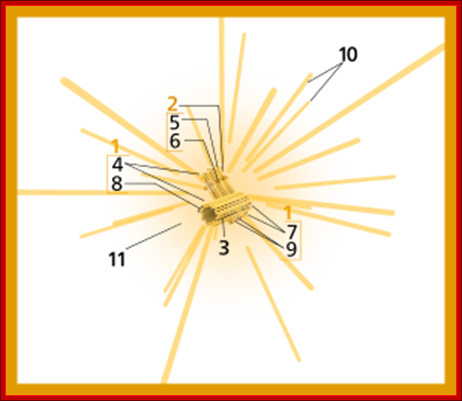
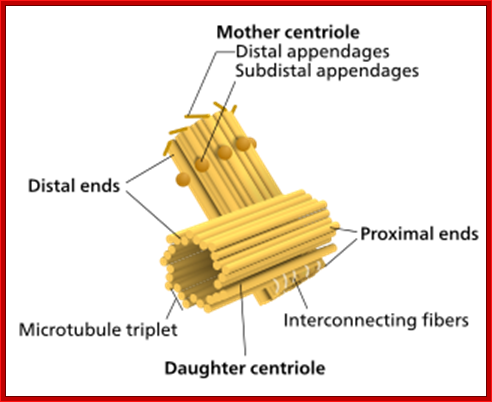
Components of a typical centrosome: 1.Centriole, 2. Mother centriole, 3. Daughter centriole, 4. Distal ends, 5. Distal appendages, 6. Subdistal appendages, 7.� Proximal ends, 8.� Microtubule triplets, 9. Interconnecting fibres, 10. Microtubules and 11. Pericentriolar material. http://commons.wikimedia.org/

a | Schematic view of the centrosome. In each triplet, the
most internal tubule is called the A-tubule; the one following it is the
B-tubule; and this is followed by the most external one, the C-tubule. At its
distal end, the centriole constitutes of doublets. Adapted with permission from
Ref.63 � (2005) Macmillan Magazines Ltd. b | Electron micrograph of the centrosome; the top inset
indicates a cross-section of sub distal appendages; the bottom inset indicates
a cross-section of the proximal part of the centriole. Note the triplet
microtubules (MTs). Scale bar: 0.2  m. Adapted with
permission from Ref. 32 � (1992) Elsevier. c | Electron micrographs and schematic view of the flagella
of green algae. There are different types of cilia and flagella, depending on
the structure of the axoneme. The axoneme is a cylindrical array of nine
doublet MTs that surround either zero MTs (called structure 9C0) or the two
singlet MTs (structure 9C2), represented here. The two singlet MTs are called
the central pair. Differences in the structure of axonemes might be reflected
in their properties: for example, whether they are motile or not (reviewed in
Ref. 137). The transition fibres extend from
the distal end of the basal body to the cell membrane. It has been suggested
that they can be part of a pore complex that controls the entry of molecules
into the cilia. Scale bar: 0.25
m. Adapted with
permission from Ref. 32 � (1992) Elsevier. c | Electron micrographs and schematic view of the flagella
of green algae. There are different types of cilia and flagella, depending on
the structure of the axoneme. The axoneme is a cylindrical array of nine
doublet MTs that surround either zero MTs (called structure 9C0) or the two
singlet MTs (structure 9C2), represented here. The two singlet MTs are called
the central pair. Differences in the structure of axonemes might be reflected
in their properties: for example, whether they are motile or not (reviewed in
Ref. 137). The transition fibres extend from
the distal end of the basal body to the cell membrane. It has been suggested
that they can be part of a pore complex that controls the entry of molecules
into the cilia. Scale bar: 0.25  m. CW, cartwheel (one of
the first structures to appear in a forming centriole). Adapted with permission
from Ref. 138 � (2002) Macmillan Magazines Ltd, and with permission from
Ref. 139� (2004) Company of Biologists.; Centriole and basal body structure. M�nica Bettencourt-Dias &
David M. Glover; http://www.nature.com/
m. CW, cartwheel (one of
the first structures to appear in a forming centriole). Adapted with permission
from Ref. 138 � (2002) Macmillan Magazines Ltd, and with permission from
Ref. 139� (2004) Company of Biologists.; Centriole and basal body structure. M�nica Bettencourt-Dias &
David M. Glover; http://www.nature.com/
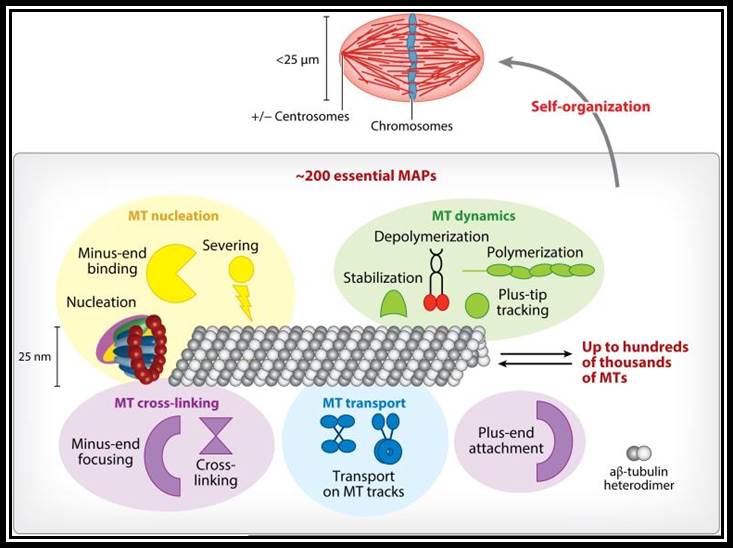
Self-organization of the metaphase spindle. The mitotic spindle is made of up to hundreds of thousands of microtubules (MTs) and roughly 1,000 MT-associated proteins (MAPs), of which ~200 are essential (22�25). Spindle MAPs can be grouped into several activity classes, namely MT nucleation, MT dynamics, MT transport, and MT cross-linking. Aside from MT nucleation by the ring-shaped γ-TuRC, the MT number can be increased by MT severing and MT minus-end-binding proteins. MT dynamics are regulated by MT polymerases, MT depolymerases, plus-end tracking proteins (+TIPs), and MT stabilization factors. MT cross-linking can occur at minus and plus ends or along the MT lattice. Finally, molecular motors are responsible for transport on MT tracks. MAPs and MTs self-organize into the functional spindle, whose maintenance continuously consumes and dissipates energy.
��������������������������������������������������������� 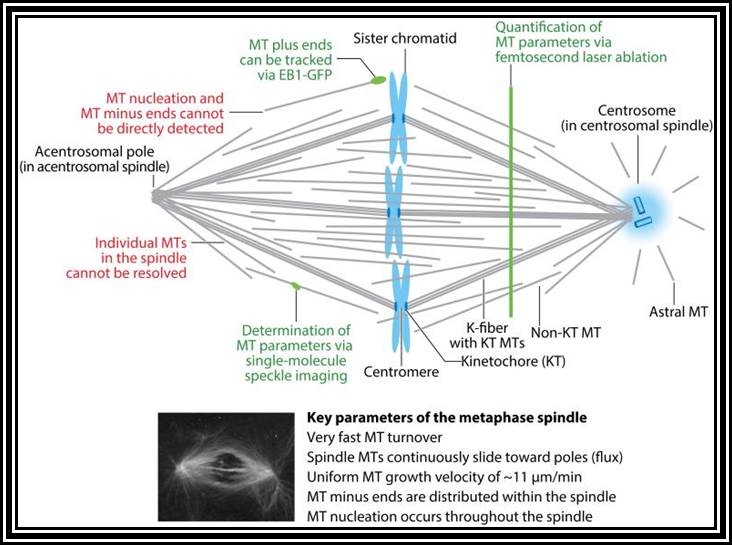
Anatomy of the metaphase spindle. To date, it has not been possible to directly determine the key parameters that describe microtubule (MT) organization within the spindle, namely the origin, dynamics, polarity, and location of each MT, presumably because the high MT density precludes their direct observation. Markers are lacking for MT minus ends and MT nucleation events, but growing MT plus ends can be tracked via the MT plus-tip tracking protein EB1-GFP (74, 75). Single-molecule speckle imaging has been applied to determine tubulin turnover and MT length distributions in the spindle (78, 79). Lastly, femtosecond laser ablation leads to MT depolymerization of newly generated plus ends, a technique used to quantify MT density and identify the locations of MT plus and minus ends (5). Altogether, these results suggest that MT nucleation combined with local MT transport govern spindle morphogenesis. Abbreviations: EB1-GFP, GFP-labeled end-binding protein 1; K-fiber, kinetochore fiber; KT, kinetochore. Annu Rev Biochem. 2016
��������������������������������������������������������� 
The microtubule scaffold and associated structures of the centriole. (a) Variations in centriole microtubule number. The top panels are Drosophila centrioles in longitudinal (left) and cross (right) section, showing doublet microtubules [6]. The lower panels are a cross section of a C. elegans centriole (left) showing singlet microtubules, and a human centriole (right) showing triplet microtubules. Bars, 100 nm. (b) Centrioles in mammalian cells. The top panel is a pair of orthogonally arranged centrioles in a centrosome. The bottom panels display cross sections of a centriole proximal region; the structural features are indicated on the right panel. Bars, 100 nm. (c) The left panel is a model of the yeast γ-tubulin complex (indicated in yellow and blues) anchoring a microtubule (grey) to a spindle pole body via a tether (brown, such as Spc110). See Kollman et al. [7] for details. The right panel is a tomographic slice of a budding yeast spindle pole body and attached spindle microtubules (as in O'Toole et al. [8]); arrows indicate the capped microtubule minus ends. Bar, 25 nm. (d). Capped minus ends are present on the A-tubule of assembling centrioles (left panel, asterisk; purple), B- and C-tubules have open ends (red, green). The A-tubule minus end cap is absent in mature centrioles (right panels). This figure is from Guichard et al. [9] with permission. Bars, 25 nm. (e). Models showing non-tubulin structures (purple) associated with the microtubule triplets (light blue) identified in cryoelectron tomograms of basal bodies in Chlamydomonas (as in Li et al. [10], left and centre panels). Left: the Y-shaped linker facing the basal body centre. Microtubule luminal structures such as the A- and B-tubule linker (arrowhead) and the A-tubule cone shaped structure (asterisk). The middle panels show microtubule luminal structures (arrows) present in the C-tubule in the distal region of the basal body. The right panel shows the location of the A-tubule to C-tubule linker (green) between microtubule triplets (purple) in Trychonympha ([11] with permission). Bar, 25nm nm. �National Library of Medicine
Centrioles� Duplication: Cells in G0 and G1 usually contain two complete Centrioles. The older of the two Centrioles in a pair is termed the mother Centriole, whereas the younger is termed the daughter Centrioles. During the cell division cycle, new Centrioles grow from the side of each of the existing "mother" Centrioles. After Centrioles duplication, the two pairs of Centrioles remain attached to each other in an orthogonal configuration until mitosis; when the mother and daughter Centrioles separate in a manner dependent upon the enzyme separase. In phase G1 the two Centrioles cylinders move very slightly apart from one another. During S phase new cylinders of microtubules form near, and at right angles to, the two �mother� cylinders. The two pairs of Centrioles keep very close to one another until the prophase stage of mitosis.� At this point they separate with both pairs of Centrioles moving over the outer surface of the nuclear envelope to opposite ends or �poles� of the cell, to form the astral poles of the dividing cell. Source(s): http://www.bscb.org/?url=softcell/centri�
The two Centrioles in the centrosome are connected to each other by unidentified proteins. The mother Centriole has radiating appendages at the distal end of its long axis and is attached to the daughter Centriole at the other proximal end. Each daughter cell formed after cell division will inherit one of these pairs (one older and one newer centriole). Duplication of Centrioles starts at the time of the G1/S transition and ends before the onset of mitosis (Wikipedia).
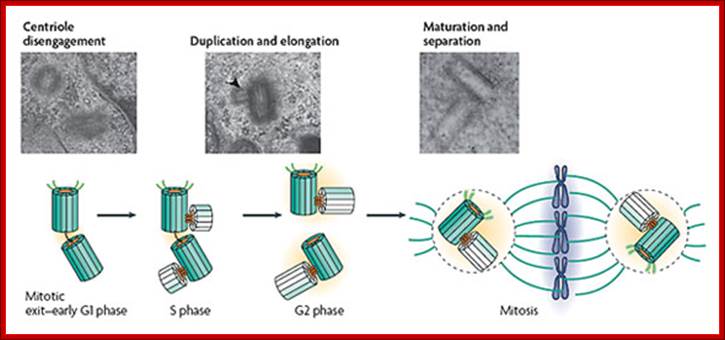
The canonical centriole duplication cycle; Electron microscopy micrographs of HeLa cells showing distinct steps of centriole duplication (also represented diagrammatically); The mother centriole is represented in dark green showing appendages. Daughter centrioles are shown in light green. At mitotic exit�early G1 phase, centrioles in a centrosome lose their orthogonal configuration. There might be an intercentriole link at this stage. Next, duplication starts in late G1�S phase with the nucleation of daughter centrioles (see electron micrograph; the arrowhead shows a procentriole). Note that the axis of the daughter intercepts the parent. The procentrioles elongate fully by late G2 phase or by the beginning of G1 phase of the next cell cycle. Last, maturation and separation of the two centrosomes occur at the G2�M transition by the acquisition of maturation markers, the recruitment of pericentriolar material (PCM; orange) and an increase in microtubule-organizing center (MTOC) activity. From Bettencourt-Dias & Glover (2007), please see review for references. Centrosome biogenesis; http://sites.igc.gulbenkian.pt/
Celia and flagella develop from mother Centrioles. http://en.wikipedia.org/
� Cilia and Flagella - For single-celled eukaryotes, cilia and flagella are essential for the locomotion of individual organisms. In multicellular organisms, cilia function to move fluid or materials past an immobile cell as well as moving a cell or group of cells.
� Primary cilium consists of 9+0 microtubules, they are found in certain cell types, epithelial linings of airways; it grows out older of the two Centrioles. The MTs extension is covered my membrane to which receptor are embedded.� The primary Celia does not beat because it lacks central MTs, they are involved in sensory reception, like mechanoreceptors, chemo receptors, photoreceptors cells.� They lack dyneins.
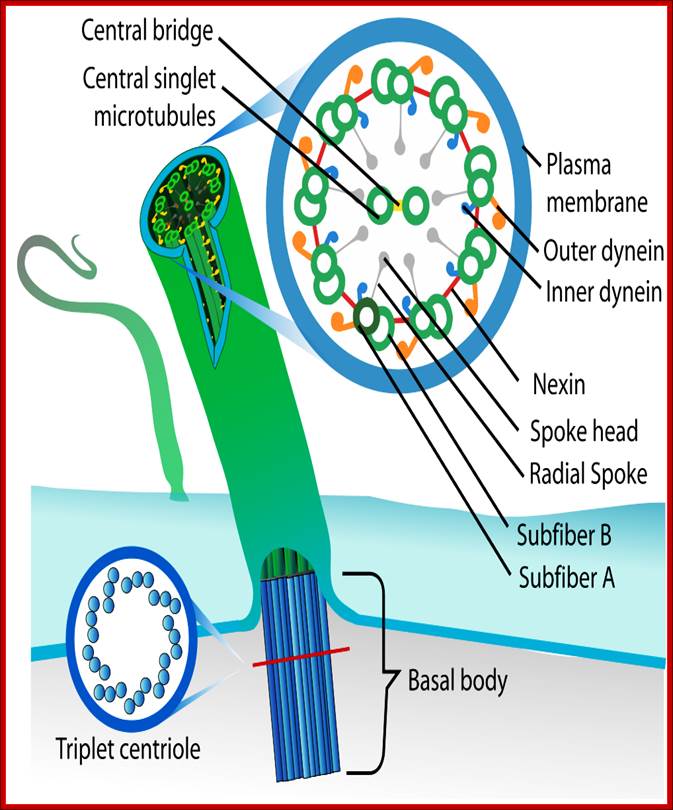
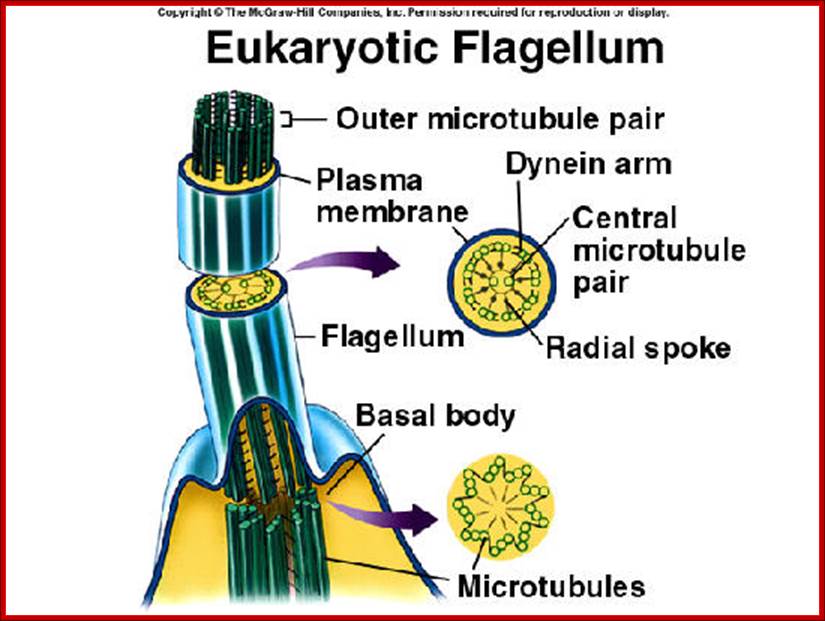
� Motile cilia consist of 9+2 MTs.� The outer ring of MTs is stuck to each other like �8� configuration, when dyneins binds to the one side of the doublet, it flexes and curves.� From outer pair of MTs a pair of Dynein arms reaches the next pair of MTs.� They hold adjacent pairs of MTs; this causes the cilia to bend.� Radial spokes extend from each of the outer MTs towards central tubules.� Nexin join each of the outer pair of MT with the adjacent outer pair.� The central pair of MTs is joined with central bridge. The cilia extend up to 5-10um. The basal body of the Cilia consists of nine triplet Centrioles in the periphery.
Structure of Celia: Eukaryotic cilia and flagella (the terms are interchangeable) come in a variety of sizes and functional roles, but are all generated by the assembly of axonemal microtubules from the centrioles of the basal body. Motile cilia are capable of periodic beating and can thus move fluid over a surface (e.g. surface of respiratory epithelia) or propel a cell (e.g. sperm). Non-motile cilia are involved in sensory signalling in, for example, the kidney epithelium, photoreceptors, and olfactory neurons. Nearly all mammalian cell types generate a non-motile cilium during the G0 and/or G1 phases of the cell cycle. This single cilium is often referred to as the primary cilium or sensory cilium. The organelle is membrane-bound and contains multiple microtubules running along its length. Whereas primary cilia have relatively little additional structure, motile cilia have both a central doublet of microtubules as well as inner and outer dynein arms and radial spokes, which are all needed for motility. � 2007 Nature Publishing Group Ainsworth, C. Cilia: Tails of the unexpected.; Nature 448, 638�641 (2007) doi:10.1038/448638a. All rights reserved. http://www-personal.umich.edu/
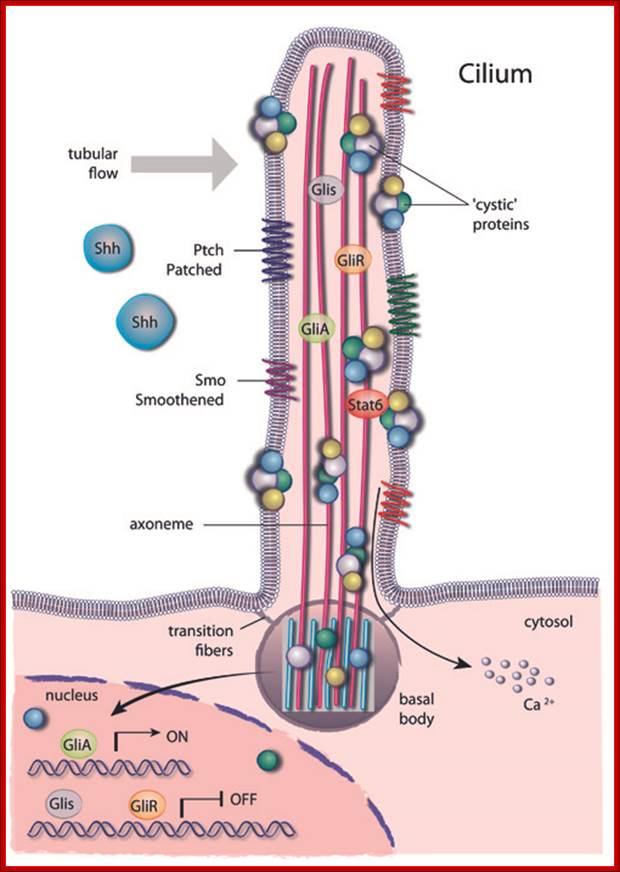
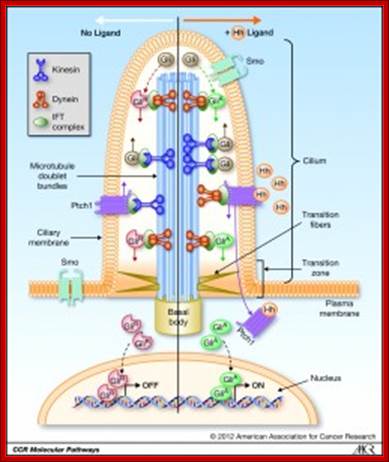
Schematic drawing of a cilium transmitting an extra-cellular signal to the nucleus; A primary cilium consists of a central axoneme made of microtubules enclosed by a distinct cell membrane.
Several structural elements such as the periciliary membrane, the transition fibres and basal bodies form a selective barrier at the entrance of the cilium and create a unique environment that allows for compartmentalization. https://www.mdc-berlin.de
http://www.cell.com
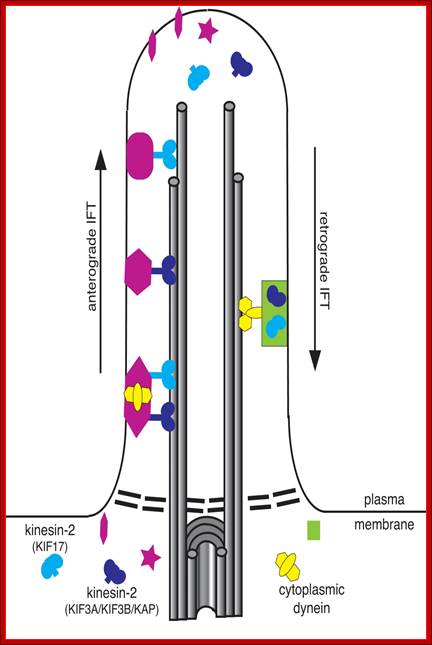
The primary apical cilium in renal epithelia. Primary cilia are hair like structures that emerge from 1 of the 2 basal bodies (centrioles) just below the apical membrane. The basal bodies are oriented perpendicular to one another, and each contains 9 microtubule triplets. The ciliary axoneme extends from the basal body and consists of microtubules arranged as 9 peripheral doublets (9+0 pattern). Along this axonemal scaffold, large protein complexes [intraflagellar transport (IFT) rafts] are transported in a bidirectional fashion. Anterograde or outward movement of IFT rafts is powered by the kinesin-II molecular motor [heterotrimeric kinesin (KIF3)], whereas the retrograde or inward movement is dependent on cytoplasmic dynein 1b/2 (reviewed in Ref. 129). Polaris, the protein disrupted in orpk mice, is an IFT raft component thought to play a critical role in ciliogenesis. Cystin, the protein truncated in cpk mice, is proposed to be associated with the ciliary membrane. The Invs protein product inversin is localized to cilia, but its intra-organelle associations remain to be defined. Both polycystin-1 (PC-1) and polycystin-2 (PC-2) localize to the primary cilia and are proposed to function in a mechanotransduction pathway. http://ajprenal.physiology.org/
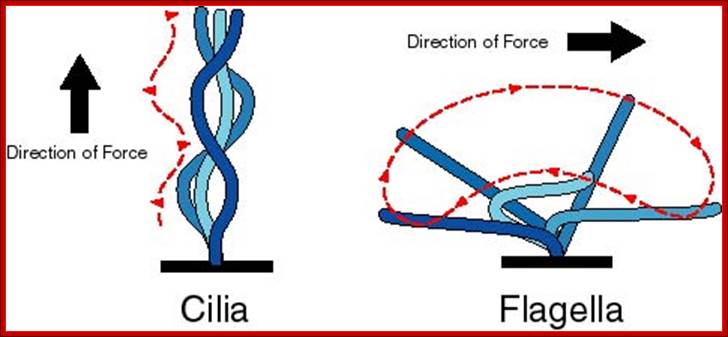
Cilia - short, usually numerous hair like projections that can move in an undulating fashion (e.g., Paramecium, lining of human upper respiratory tract); Flagella - longer, usually fewer, whip-like projections that move in whip-like fashion (e.g., sperm cells); http://bioserv.fiu.edu/
Celia and flagella are involved in cell movement. Both are composed of microtubules. Celia are short and numerous and complex. Flagella are longer, fewer and less complex. Both are organized in 9+2 pattern with dynein arms projecting outward. http://www.uic.edu/
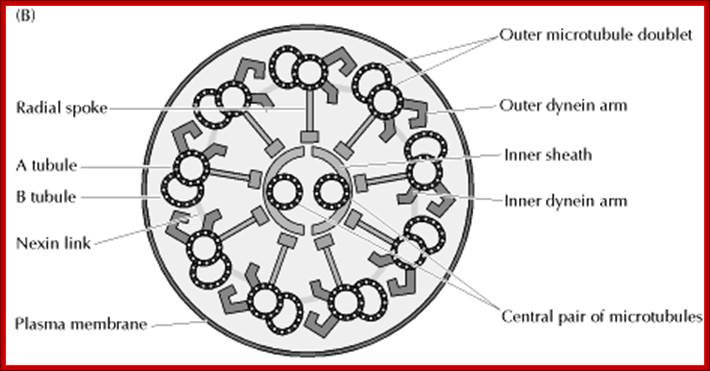
Structure of the axoneme of cilia and flagella;
Eukaryotic cilia and flagella are very similar structures, each with a diameter of approximately 0.25 μm. Many cells are covered by numerous cilia, which are about 10μm in length. Cilia beat in a coordinated back-and-forth motion, which either moves the cell through fluid or moves fluid over the surface of the cell. For example, the cilia of some protozoans (such as Paramecium) are responsible both for cell motility and for sweeping food organisms over the cell surface and into the oral cavity. In animals, an important function of cilia is to move fluid or mucus over the surface of epithelial cell sheets. A good example is provided by the ciliated cells lining the respiratory tract, which clear mucus and dust from the respiratory passages. Flagella differ from cilia in their length (they can be as long as 200μm) and in their wavelike pattern of beating. Cells usually have only one or two flagella, which are responsible for the locomotion of a variety of protozoans and of sperm. http://kc.njnu.edu.cn/
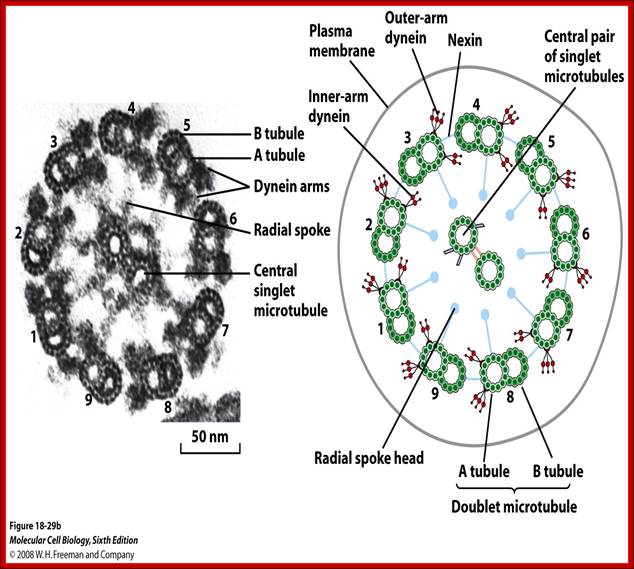
Eukaryotic cilia and flagella consist of a "9+2" arrangement of microtubules connected by Dynein, which uses ATP to create a wave like bending movement; http://www.bio.miami.edu/
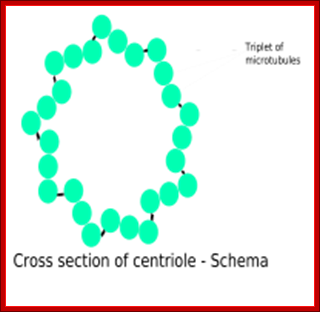
http://biobook.nerinxhs.org/
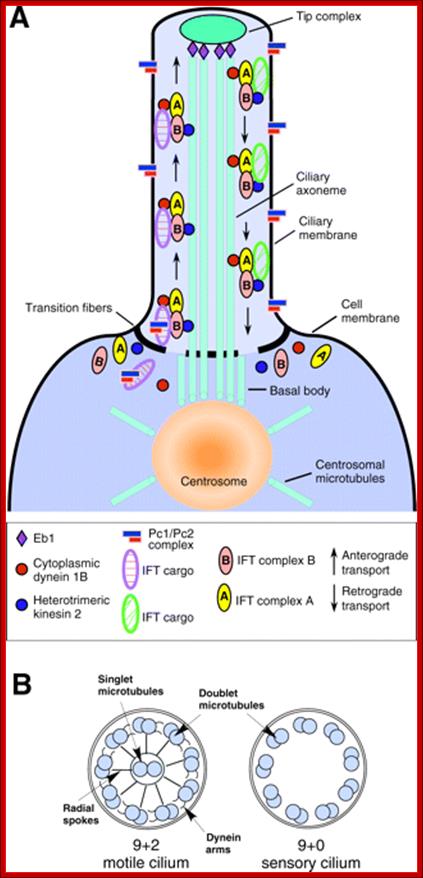
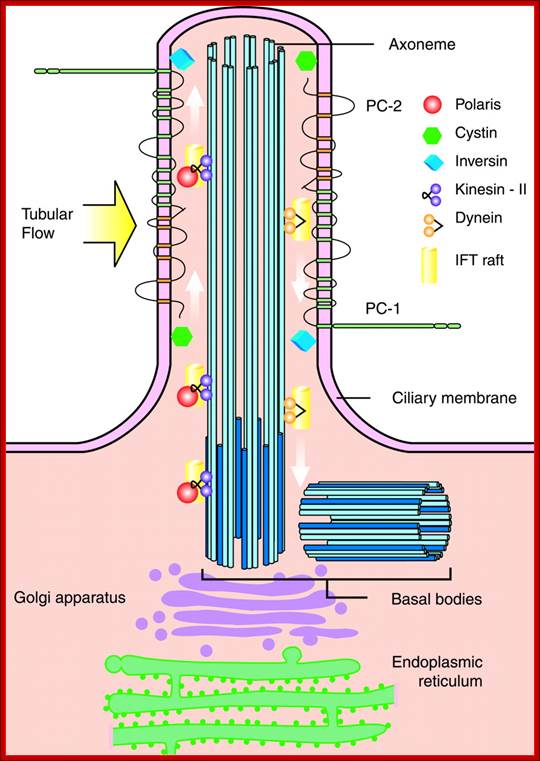
Ciliary base and the number of microtubule bundles doublets and singlets; Cilia structure and intraflagellar transport. (A) A typical cilium consists of an axoneme of nine doublet microtubules (two are shown in B). Each doublet arises from the inner two microtubules of the basal body microtubule triplets. The axoneme is surrounded by a specialized ciliary membrane that is separated from the cell membrane by a zone of transition fibers. (B) A cross-section of 9+2 and 9+0 cilium. Cilia are broadly divided into two types based on the presence or absence of a central pair of microtubule singlets in the axoneme (9+2 or 9+0 structure, respectively). Inner and outer dynein arms, which are usually associated with 9+2 cilia, can be present in either type of cilium and are important for ciliary motility. Ciliary assembly and maintenance is accomplished by intraflagellar transport (IFT), which relies on the microtubule motor proteins kinesin 2 and cytoplasmic dynein to transport IFT protein complexes and their associated cargo up and down the length of the cilium (depicted in A). Abbreviations: Eb1, end-binding protein 1; Pc1 and Pc2, polycystin 1 and polycystin 2. http://dev.biologists.org/cgi/content-nw
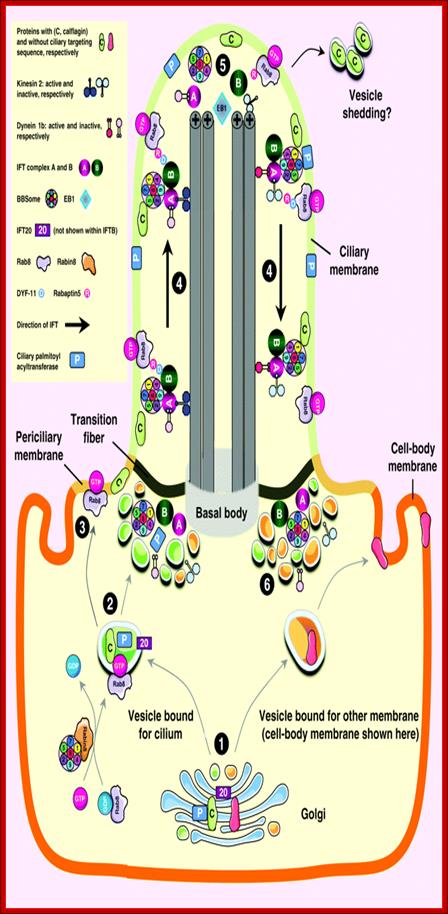
Left diagram; Cilia structure and intraflagellar transport. (A) A typical cilium consists of an axoneme of nine doublet microtubules (two are shown in B). Each doublet arises from the inner two microtubules of the basal body microtubule triplets. The axoneme is surrounded by a specialized ciliary membrane that is separated from the cell membrane by a zone of transition fibers. (B) A cross-section of 9+2 and 9+0 cilium. Cilia are broadly divided into two types based on the presence or absence of a central pair of microtubule singlets in the axoneme (9+2 or 9+0 structure, respectively). Inner and outer dynein arms, which are usually associated with 9+2 cilia, can be present in either type of cilium and are important for ciliary motility. Ciliary assembly and maintenance is accomplished by intraflagellar transport (IFT), which relies on the microtubule motor proteins kinesin 2 and cytoplasmic dynein to transport IFT protein complexes and their associated cargo up and down the length of the cilium (depicted in A). Abbreviations: Eb1, end-binding protein 1; Pc1 and Pc2, polycystin 1 and polycystin 2. Kinesin 2 moves the IFT Intra flagellar Transport complex and its cargo (e.g., Gli, Ptch, and Smo) toward the plus end of microtubules (ciliary tip). Dynein 2 moves the IFT complex and its cargo toward the minus end of microtubules (cell body).
Right diagram: Protein targeting to the ciliary membrane. The eukaryotic cilium is a distinct organelle that is separated from the cytoplasm by transition fibers that connect the basal body to the membrane and separate the ciliary membrane (green) from the periciliary (pale orange) (Reiter and Mostov, 2006) and cell-body membranes (dark orange) (Sloboda and Rosenbaum, 2007). Although cilia in certain cell types differ in the fine details of their structures, functions, mechanisms of assembly and regulation, some general principles have emerged in recent years; this figure attempts to integrate these general concepts. The ciliary membrane has a lipid composition that is distinct from that of the periciliary and cell-body membranes because it is highly enriched in sterols, glycolipids and sphingolipids (Tyler et al., 2009). This specialized composition is probably formed in the Golgi (Ejsing et al., 2009; Schuck and Simons, 2004). http://jcs.biologists.org/
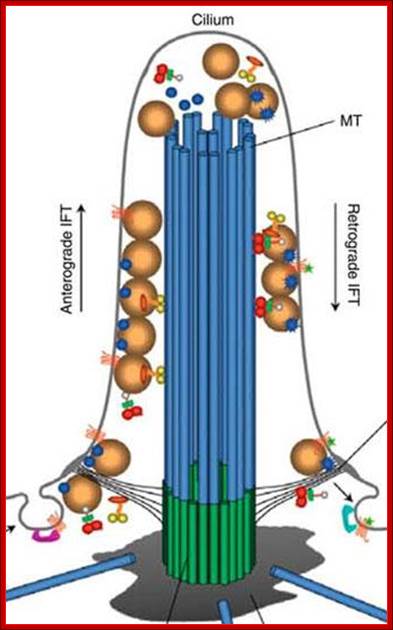 �
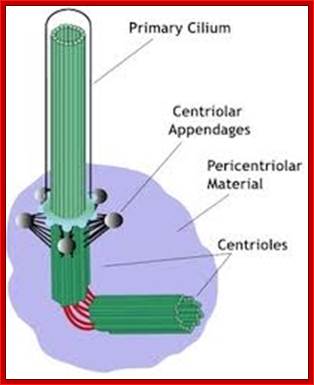
�
In 1993 the intra-flagella transport system IFT in the PI was observed and connected to polycystic kidney disease. It was observed that the microtubule structure, called axoneme, grows from the base of the PI, called the basal body. When the cilium is being built, vesicles transport protein pieces to the growing cilium from the base.
Recently, the complexity of the transport system has been described. A special protein attaches and drags proteins through the sea of phospholipids in the membrane, and pulls them into the PI. In this way the primary cilium becomes the communication hub of the cell. Already a number of critical signaling cascades have been shown to live in the primary cilium. This includes the critical hedgehog and retinal signaling pathways.
The transport system uses motors that travel along microtubules to get the important material to the tip of the PI from the base. Special motors are built at the base of the PI and they pull many different types of material into the PI�receptor proteins and building blocks for microtubules. Once at the tip of the PI, the motors deposit the cargo. T the tip the motor is altered and becomes a different machine to bring signaling material down the PI to the base. At the based messages are created and sent to the nucleus. - See more at: http://jonlieffmd.com/blog/is-the-primary-cilium-a-cells-antenna-or-its-brain#sthash.3YF26Ogj.dpuf
Once at the base of the PI, the motor rearranges itself and becomes the train that drives cargo up into the PI. The train that pulls this material to the tip of the PI is made of at least four motors, one type active at a time. These motors are not just motors; they also interact with the membrane to regulate other functions including sensing extracellular situations and influencing decisions during fetal development. These motors are also able to connect through the membrane to objects outside of the cell to stimulate different types of cell movement. In this situation the motor is anchored to particular spot, but the entire cell moves when the motor is turned on.
This very complex motor system is critical for the elaborate function of the PI by transporting all receptors and signalling materials that are used for the antenna function.
Defects in these motors in the eye can cause blindness. In the cells of the eye, the tip of the PI is very large bulb and houses the sensors that respond to light but still has a narrow area connecting with the large cell body. All of the light signals have to go through this narrow tube and a defect leads to blindness. Proteins used for sensing light are fragile and very active and many are imperfect. The cell is very dependent on the transport system of the PI to continually restock the proteins. Retinitis pigmentosa is one of the many diseases (ciliopathies) related to defects in this transport system.
See more at: http://jonlieffmd.com/blog/is-the-primary-cilium-a-cells-antenna-or-its-brain#sthash.3YF26Ogj.dpuf; http://jonlieffmd.com/
![]() �
�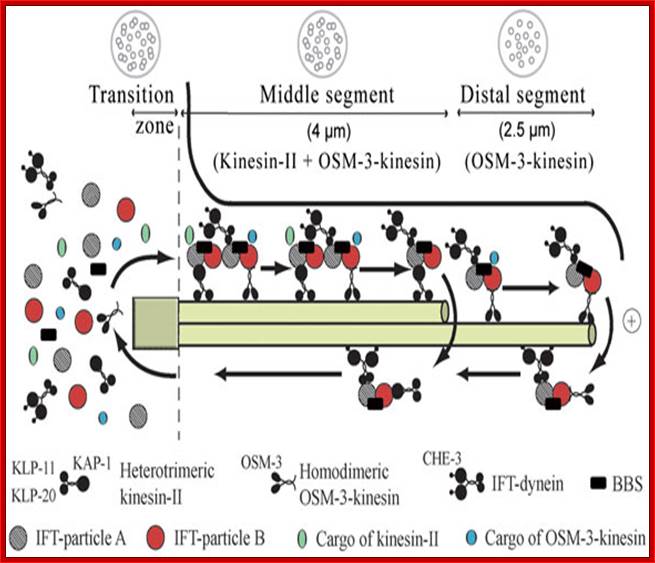
Intraflagellar transport in C. elegans; Intraflagellar transport in C. elegans; Components of the IFT machinery and ciliary cargo assemble at or near the transition zone (basal body). Two kinesins, heterotrimeric kinesin-II and homodimeric OSM-3-kinesin, separately bind IFT particle subcomplexes A and B, respectively, and transport these together with IFT-dynein and cargo along the middle segment in the anterograde (+) direction. In the distal segment, OSM-3-kinesin alone transports the IFT particles and dynein/cargo. BBS proteins act to stabilize the association between the motors and IFT particle subcomplexes A and B. Components of the IFT machinery and presumably other ciliary molecules are recycled back to the base of the cilium using the IFT-dynein molecular motor. The lengths of the transition zone (1 μm), middle segment (4 μm) and distal segment (2.5 μm) regions are shown (for amphid cilia) along with transverse view schematics of the microtubule arrangements (on top); (taken and modified from www.wormbook.org; Peter N. Inglis et al. Dr. Oliver Wagner, �http://life.nthu.edu.tw
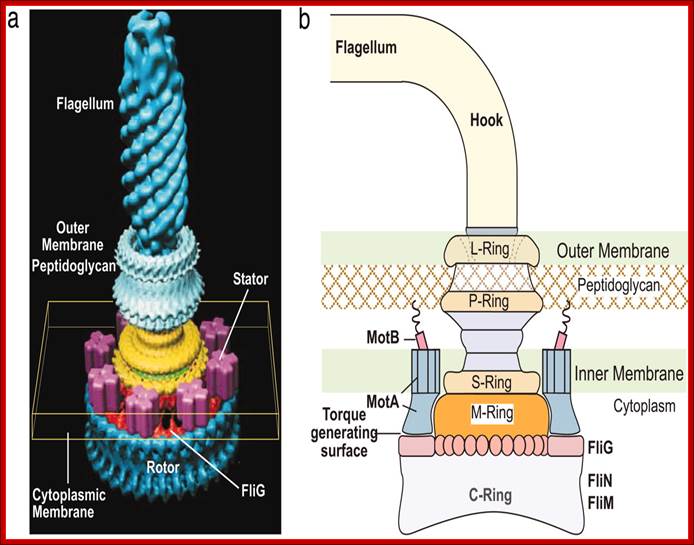
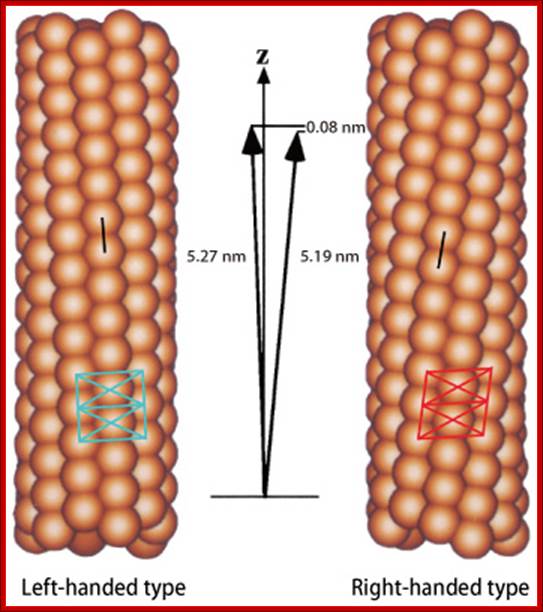
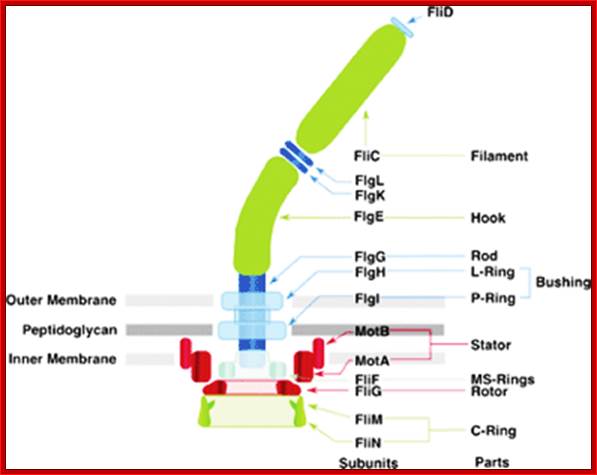
Flagella- Bacterial flagillar filament is constructed from 11 proto-filaments of flagellin protein subunits. Flagellum is 20nm thick hollow tube, it is helical, but it has a sharp bend at outer surface of the membrane.� A shaft runs between the hook and the basal body, passing through protein rings in the cell's membrane that act as bearings. Gram-positive organisms have 2 of these basal body rings, one in the peptidoglycan layer and one in the plasma membrane. Gram-negative organisms have 4 such rings: the L ring associates with the lipopolysaccharides,�� the P ring associates� with peptidoglycan layer, the M ring is embedded in the plasma membrane, and the S ring is directly attached to the plasma membrane. The filament ends with a capping protein.
Bacterial flagellum-Tubular Flagillin polymer; http://www.ks.uiuc.edu/
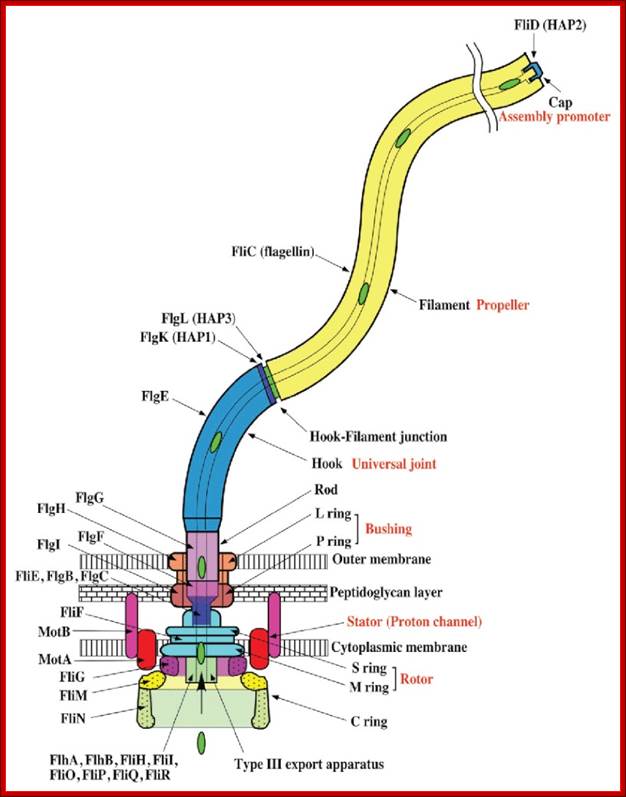
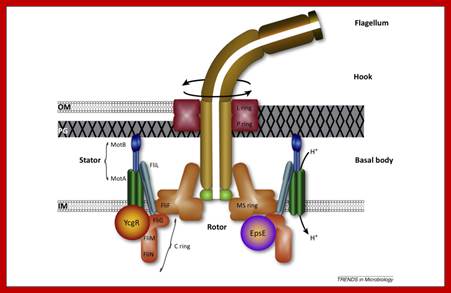
Schematic diagram of the bacterial flagellum; Different colors represent different protein components (Reprinted with permission from Yonekura et al. Res Microbiol 2002; 153:191-197. �2002, Elsevier). A putative complex of the three switch proteins, FliG, FliM and FliN appears to be directly involved in torque generation and control of direction of rotation. http://www.fbs.osaka-u.ac.jp/
Bacterial flagella are made up of Flagillin subunits; it is Nano-machine involved in bacterial movement.� A membrane embedded motor protein propels bacterium. Each flagellum consists of ~3000 Flagillin proteins (FlgE and FliC) and can grow to the length of 15um.� The hook is made up of 130 copies of FlgE subunits (~55-60nm).� Flagellar proteins are synthesized in the cell and transported to the distal or growing part of the filament through a narrow 20-25A wide central channel, a kind of secretion system in bacteria.
This supra-molecular structure is made up of three structural components-the basal body, the hook and the filament. The movement of flagella shows straight and tumble features. The bacterial flagellum is a self-assembling filament, which bacteria use for swimming. It is built from tens of thousands of Flagillin monomers in a self-assembly process that involve translocation of the monomers through the flagella interior, a channel, to the growing tip. Flagellum monomers are pumped into the filament at the base; move unfolded along the channel and then binds to the tip of the filament, thereby extending the growing flagellum. The Flagillin translocation process, due to the flagellum maximum length of 20 μm, is an extreme example of protein transport through channels.
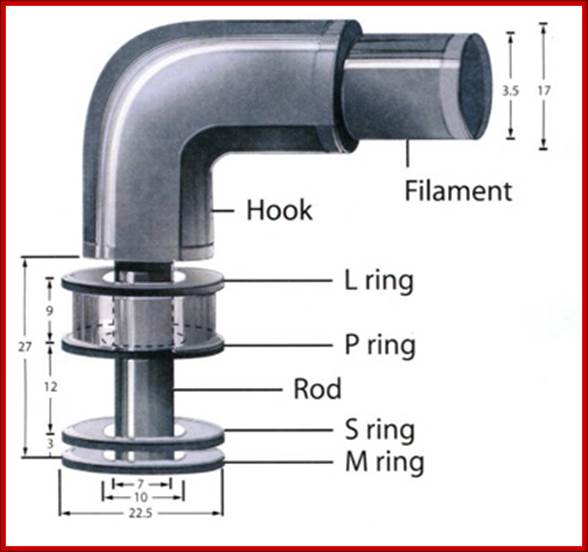
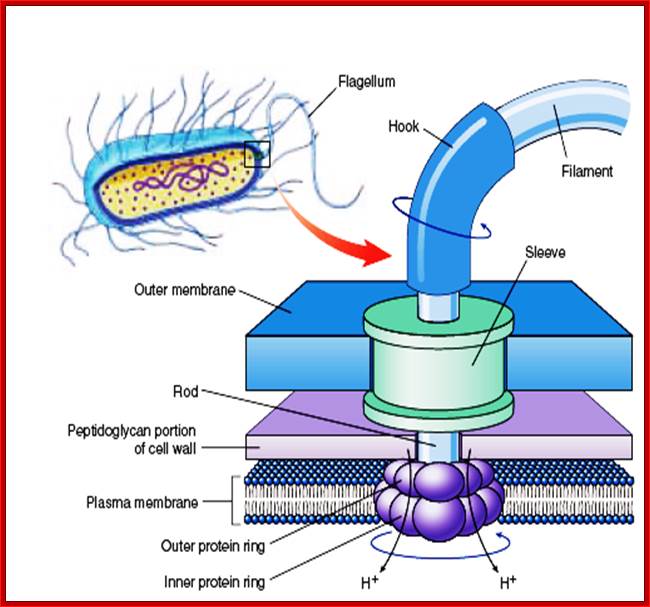
Figure: The ultrastructure of a bacterial flagellum (after J. Adler). Measurements are in nanometers. The flagellum of E. coli consists of three parts, filament, hook and basal body, all composed of different proteins. The basal body and hook anchor the whip-like filament to the cell surface. The basal body consists of four ring-shaped proteins stacked like donuts around a central rod in the cell envelope. The inner rings, associated with the plasma membrane, are the flagellar powerhouse for activating the filament. The outer rings in the peptidoglycan and outer membrane are support rings or "bushings" for the rod. The filament rotates and contracts which propels and steers the cell during movement. Bacterial flagella is made up of Flagillins. http://stiintasitehnica.com/
����������������� A physical model of bacterial flagellum; http://jonlieffmd.com/
TS of Bacterial flagella. �http://galleryhip.com/
Flagellar cylinder
Eukaryotic Flagella:
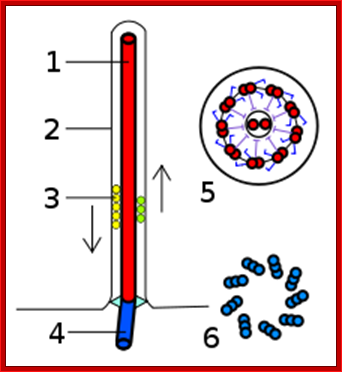
Fig-Eukaryotic flagella- central axonema 9+2 MT doublets,Two central MTs, peripheral membrane, granular IFT (intra flagellar transport) and bask body. Eukaryotic flagella. 1-axoneme, 2-cell membrane, 3-IFT (intraflagellar transport), 4-basal body, 5-cross section of flagella, 6-triplets of microtubules of basal body. http://en.wikipedia.org/
Lab on a chip: http://pubs.rsc.org/
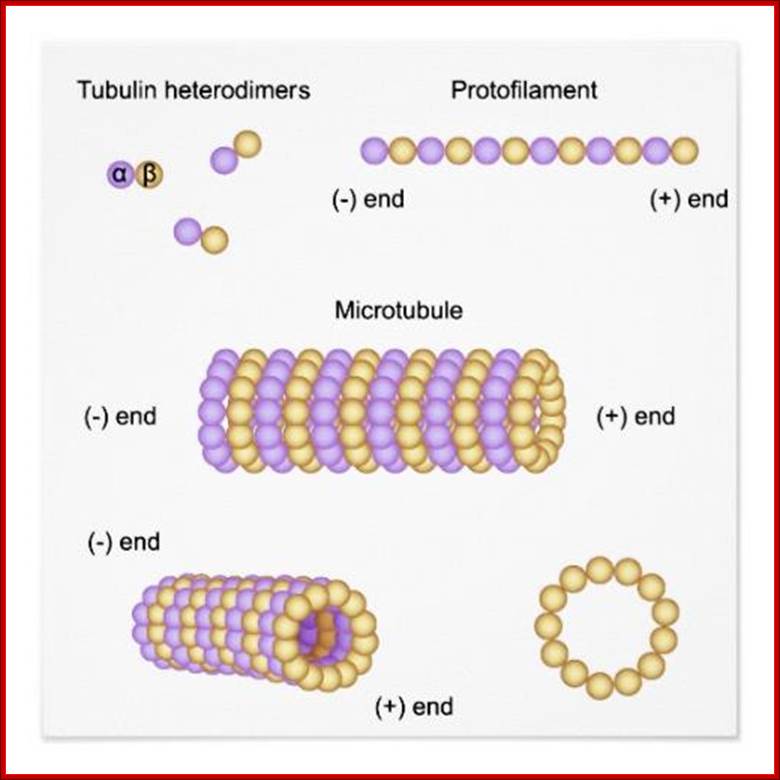
Microtubules one of the important cytoskeletons, consists of alpha and beta tubulins which are associated to form dimmers as units.� These in turn associate from the basal centriole apparatus into extended tubular filament called flagellum.� This structure grows from (-) end toward (+) end, where new tubulin dimmers are added.
Doublet and triplet microtubules, flagellar dynein motors, and the 9+2 microtubule architecture common to these organelles. The last common ancestor of all eukaryotic organisms possessed a 9+2 flagellum that was used for gliding motility along surfaces, beating motility to generate fluid flow, and localized distribution of sensory receptors, and trace possible earlier stages in the evolution of these characteristics. http://faculty.southwest.tn.edu
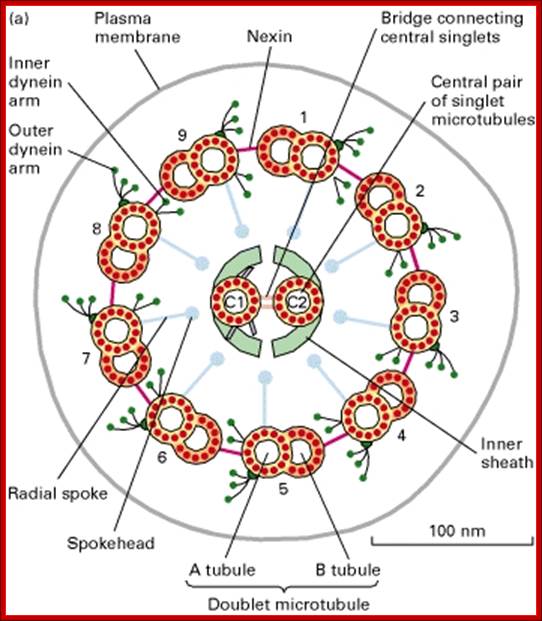
Figure-Structure of Flagellar axonemes:
(a) Cross-sectional diagram of a typical flagellum showing its major structures. The dynein arms and radial spokes with attached heads occur only at intervals along the longitudinal axis. The central microtubules, C1 and C2, are distinguished by fibers bound only to C1. (b) Micrograph of a transverse section through an isolated demembranated cilium. The two central singlet microtubules are surrounded by nine outer doublets, each composed of an A and a B subfiber. [Part (b) courtesy of L. Tilney; see U. W. Goodenough and J. E. Heuser, 1985, J. Cell Biol. 100:2008.
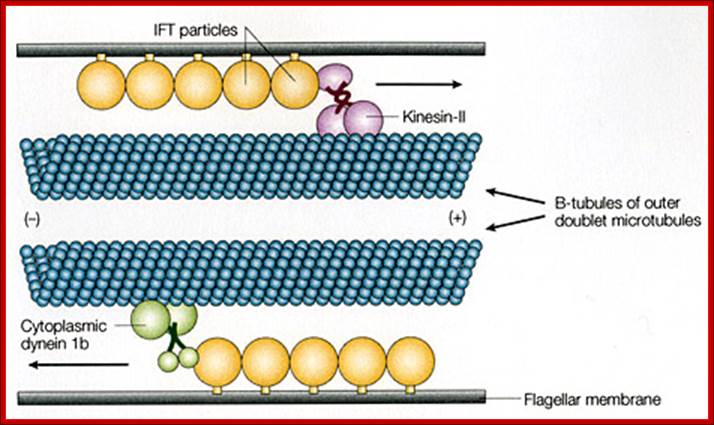
Interflagellar transport machinery found associated with outer boundary of cylindericl MTs resposnsible for� transport of cargo including membrane proteins.� The transport is facilitated from (-) end towards (=) end by kinesins II motor proteins.� But Dynein motor proteins transport cargo from (=) end towards (-) end.� IFTS a complex of 19 different proteins to be carrying precursors essential for assembly flagellar axoneme.. IFTs are linked to membranes. https://profiles.umassmed.edu
https://profiles.umassmed.edu
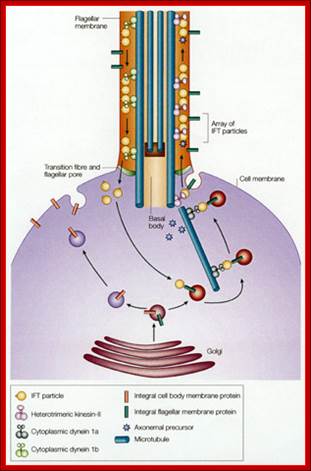
FT and targeting of proteins to the flagellar compartment. Flagellar membrane proteins are carried by vesicles from the Golgi apparatus to the base of the flagellum, where they fuse with the plasma membrane of the cell. In this figure, proteins destined for the flagellar membrane are sorted into specific vesicles that are then targeted to the base of the flagellum. This sorting and targeting appears to be aided by one or more IFT-particle proteins that cycle from the base of the flagellum back through the endomembrane system, where they become associated with the proteins that are destined for the flagellar membrane. Once the vesicle is exocytosed, the IFT-particle proteins, with attached flagellar membrane proteins, become incorporated into IFT particles and are moved through the flagellar pore (involving outer doublet-membrane links in the flagellar transition zone) into the flagellar compartments.
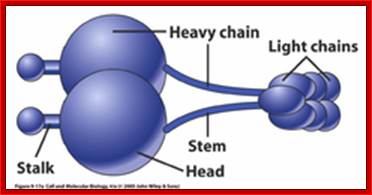
Dyneins- Five main parts of Dyneins- Stalk, head, heavy chai, stem and Light cahin; ~1000kDa, and there are different kinds such as cytoplasmic and axonemal; dyneins move in (-) end direction; act as carrier protein of the cargo.
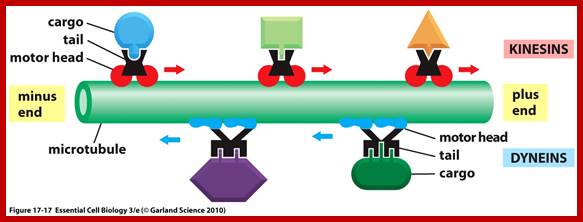
Cytoskeleton;http://oregonstate.edu http://www.biomedcentral.com/content/figures/1471-2105-7-S1-S6-1.jpg
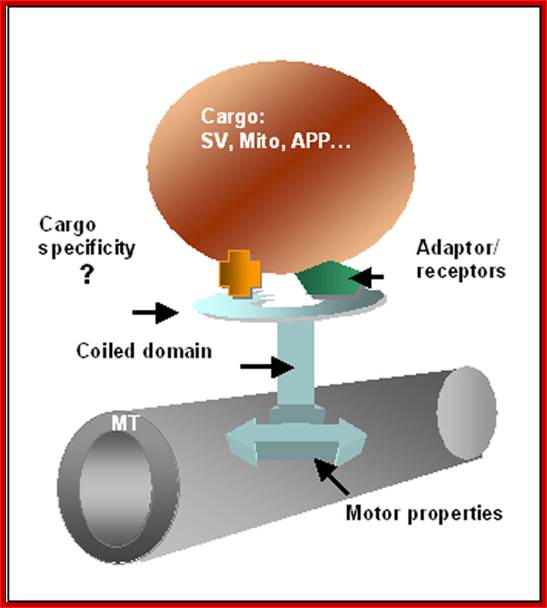
A scheme for kinesin attached to mictotubules (MT) and to a vesicle. The molecule is composed of two heads (marked by an arrow) that allow attachment to MT, a central coiled region and a region which connects the molecule to the intracellular vesicle to be moved. The movement is based on ATP hydrolysis by the head motor domain. Adaptor and receptors associated with the cargo vesicles (SV, synaptic vesicle; Mito, mitochondria; APP, Amyloid precursor protein) provide another strategy to enrich motor-cargo combinations. Linial BMC Bioinformatics 2006 7(Suppl 1):S6
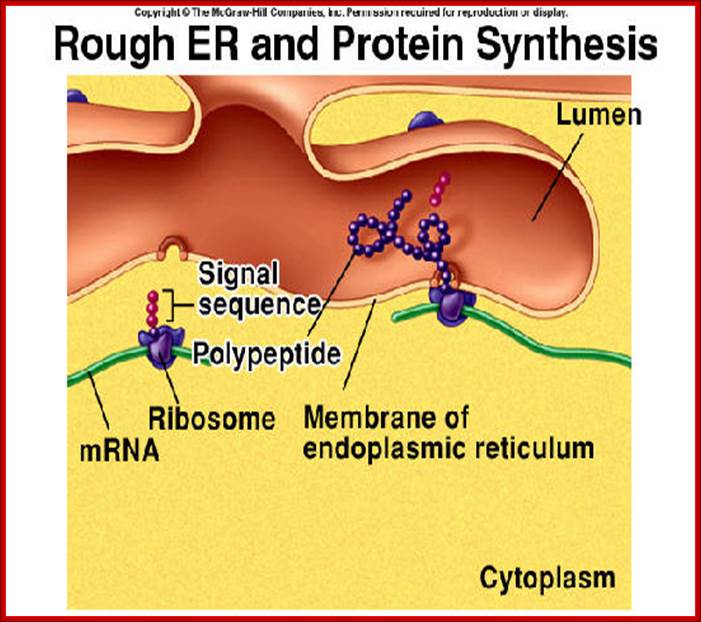
� Endoplasmic Reticulum - The endoplasmic reticulum is a network of sacs that manufactures, processes, and transports chemical compounds for use inside and outside of the cell. It is connected to the double-layered nuclear envelope, providing a pipeline between the nucleus and the cytoplasm.
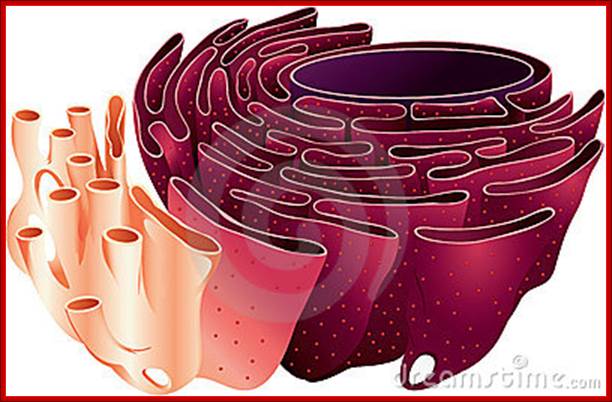
����������������������� RER and SER;� http://johnflory.files.wordpress.com/
����������������������������������������������� http://fineartamerica.com/
Endoplasmic Reticulum is pervasive cell internal region and has many important functions. RER is involved ribosome mediated protein transport into ER lumen where the protein is modified and folded and transported to Golgi complex through SER.� Often unfolded or not properly folded proteins are transported back into cytoplasm.
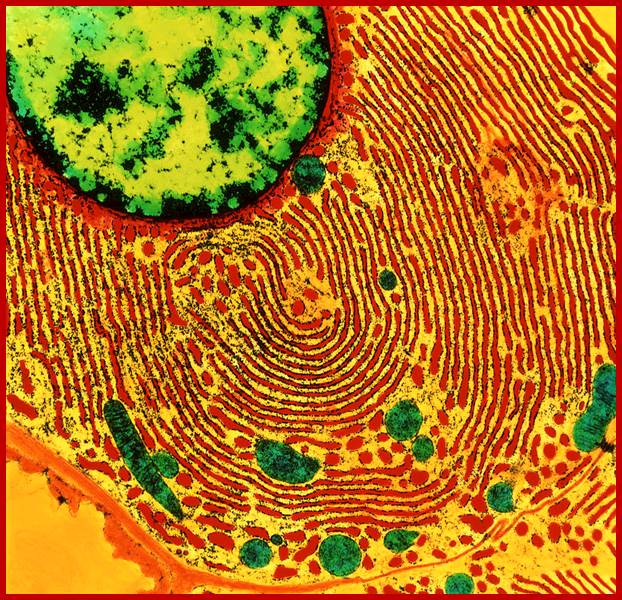
vvvvhttp://faculty.southwest.tn.edu/rburkett/Cell
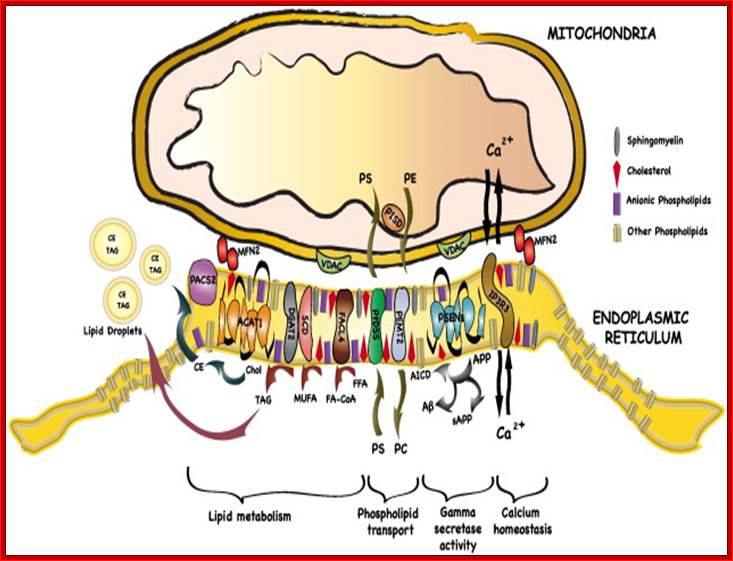
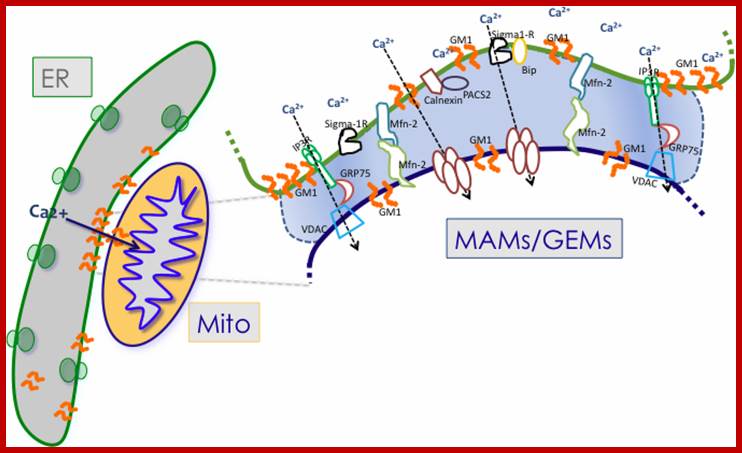
Mitochondria associated endoplasmic reticulum membrane (MAM). Schematic representation of some of the proteins and lipids localized to the MAMs. Example of proteins localized to the MAMs (such as Mfn2, Sigma 1R, BiP, IP3R1, VDAC, and GRP75) that have been shown to regulate Ca2+ signaling and the topology of ER-mitochondria microdomains. GM1 is the only lipid at the MAMs, which influences both Ca2+ flux and the number of contact sites. http://www.mdpi.com/
The dynamic interplay among intracellular organelles occurs at specific membrane tethering sites, where two organellar membranes come in close apposition but do not fuse. Such membrane microdomains allow for rapid and efficient interorganelle communication that contributes to the maintenance of cell physiology. Pathological conditions that interfere with the proper composition, number, and physical vicinity of the apposing membranes initiate a cascade of events resulting in cell death. Membrane contact sites have now been identified that tether the extensive network of the endoplasmic reticulum (ER) membranes with the mitochondria, the plasma membrane (PM), the Golgi and the endosomes/lysosomes. Thus far, the most extensively studied are the MAMs, or mitochondria associated ER membranes, and the ER-PM junctions that share functional properties and crosstalk to one another. Specific molecular components that define these microdomains have been shown to promote the interaction in trans between these intracellular compartments and the transfer or exchange of Ca2+ ions, lipids, and metabolic signaling molecules that determine the fate of the cell.
The MAM has unique phospholipid profile and involved in Ca2+ signalling and involved in inter-membrane transport and inter organelle communication.
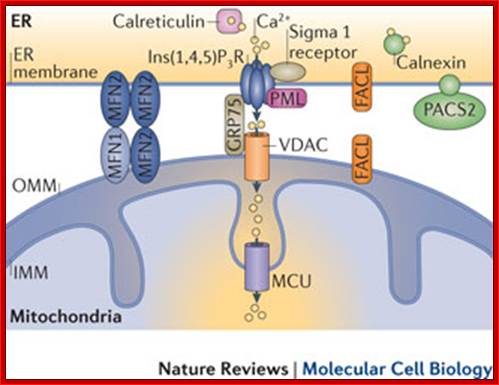
Mitochondria as sensors and regulators of calcium signalling; Close interactions between the endoplasmic reticulum (ER) and mitochondria are essential for rapid and sustained Ca2+ uptake by mitochondria. Voltage-dependent anion channels (VDACs), located at the outer mitochondrial membrane (OMM), are responsible for the rapid transfer of Ca2+ from the ER�mitochondria apposition, and their function results in high Ca2+ microdomains in the mitochondria intermembrane space. Accumulation of Ca2+ into the mitochondrial matrix occurs via the mitochondrial Ca2+ uniporter (MCU), which rapidly accumulates Ca2+ across the steep electrochemical gradient. A number of chaperones and regulatory proteins control the formation of the ER�mitochondria junction, the clustering of signalling proteins and their modulation. Mitofusin 2 (MFN2) is involved in both mitochondrial fusion and in ER�mitochondria tethering, by both homotypic interactions and heterotypic interactions with MFN1. Chaperones modulate ER Ca2+ buffering (for example, calreticulin and calnexin) and control the stability or the sorting of signalling proteins. For example, sigma 1 receptor stabilizes inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) receptors (Ins(1,4,5)P3Rs) when ER Ca2+stores are depleted, thereby ensuring proper Ca2+ fluxes from the ER to the mitochondria. Phosphofurin acidic cluster sorting protein 2 (PACS2) controls the translocation of calnexin from the ER to the plasma membrane and thereby modulates ER Ca2+ buffering and controls ER�mitochondria appositions during apoptosis. Moreover, chaperones affect the activity of ion channels. For example GRP75 (75 kDa glucose-regulated protein), which mediates the interaction of VDAC1 with Ins(1,4,5)P3R, facilitates mitochondrial Ca2+ uptake, and PML (promyelocytic leukaemia) protein, which regulates Ins(1,4,5)P3R-mediated Ca2+ release from the ER, supports mitochondrial Ca2+ uptake and thus has a crucial role during apoptosis. The family of long-chain fatty-acid CoA ligases (FACL) is involved in lipid metabolism and is enriched in mitochondria-associated membranes (MAMs). IMM, inner mitochondrial membrane. http://www.nature.com/
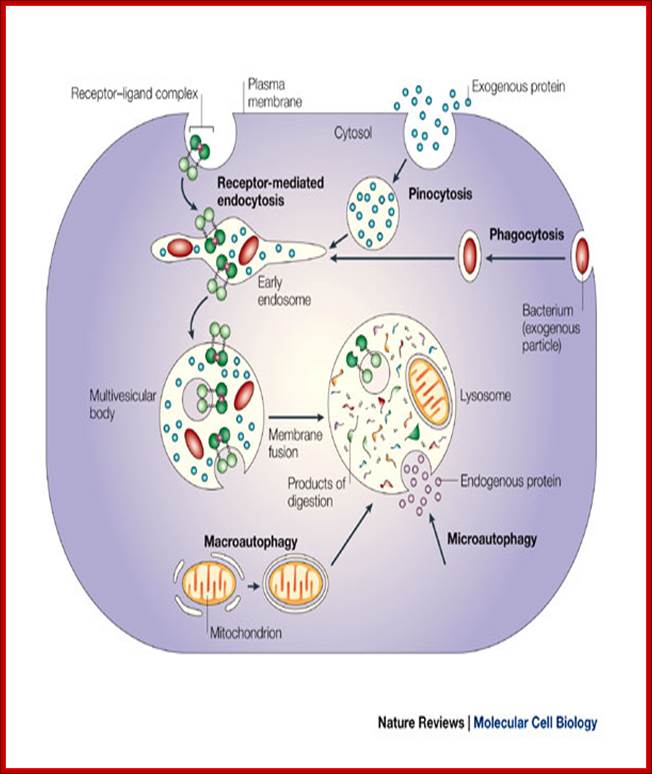
� Endosomes and Endocytosis - Endosomes are membrane-bound vesicles, formed via a complex family of processes collectively known as endocytosis, and found in the cytoplasm of virtually every animal cell. The basic mechanism of endocytosis is the reverse of what occurs during exocytosis or cellular secretion. It involves the invagination (folding inward) of a cell's plasma membrane to surround macromolecules or other matter diffusing through the extracellular fluid.
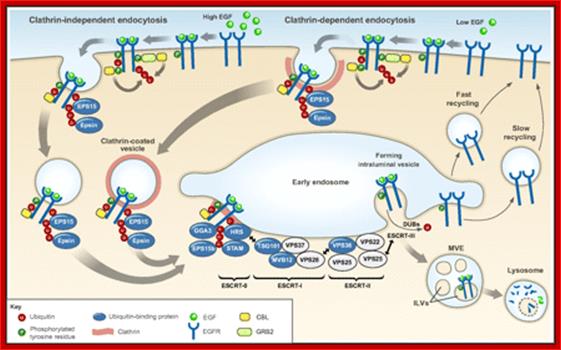
������������������������������������������ Ubiquitylation in receptor endocytosis and endosomal sorting. Following ligand binding, signaling receptors (in this example the EGFR), can undergo clathrin-mediated endocytosis (right) or clathrin-independent endocytosis (left) (Goh et al., 2010; Scita and Di Fiore, 2010; Sigismund et al., 2005). In both cases, receptors are routed to early endosomes, from where they can be sorted into ILVs of the MVE and subsequently targeted for lysosomal degradation (ubiquitylated receptors) or recycled to the plasma membrane (non-ubiquitylated receptors). As described in the main text, the contribution of ubiquitylation to EGFR endocytosis seems to depend on the experimental system used (Goh et al., 2010; Madshus and Stang, 2009; Sigismund et al., 2005). The CBL family of ubiquitin ligases has a key role in mediating RTK ubiquitylation. CBL associates with activated receptors either directly or indirectly, for example through GRB2. Ubiquitin is an essential signal for endosomal sorting of EGFRs into the ILVs of MVEs. Components of the endocytic machinery, including EPS15, epsins, the ESCRT-0 components HRS and STAM, the ESCRT-I components TSG101 and Mvb12p (in yeast) (and the novel ESCRT-I component UBAP1), the ESCRT-II component VPS36 (EAP45), as well as EPS15b and GGA3 contain ubiquitin-binding domains and have been implicated in recognizing and sorting ubiquitylated receptors either at the plasma membrane or at endosomes, as indicated in the figure. The ESCRT-I and -II components assemble in supercomplexes and have been proposed to organize buds at the endosomal membrane. The ESCRT-III complex associates with ESCRT-II and forms polymers that drive membrane scission and ILV biogenesis. DUBs catalyze the removal of ubiquitin from receptors before their translocation into the ILV, without allowing cargo to escape.;Kaisa Haglund1,* and Ivan Dikic ;;http://jcs.biologists.org/
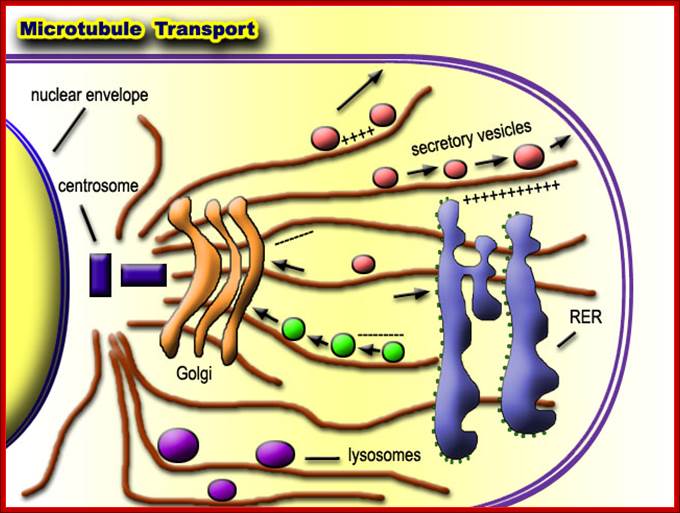
� Golgi Apparatus - The Golgi apparatus is the distribution and shipping department for the cell's chemical products. It modifies proteins and a fat built in the endoplasmic reticulum and prepares them for export to the outside of the cell. Transportation and loading and presenting antigens by MHC complexes.
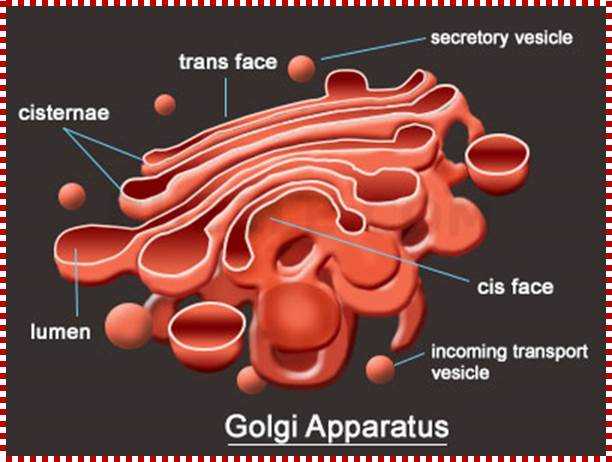
Golgi apparatus is a very important organelle; proteins that are transferred to cis golgi are sorted and packed and released as vesicle, that reach various destinations. http://www.buzzle.com/
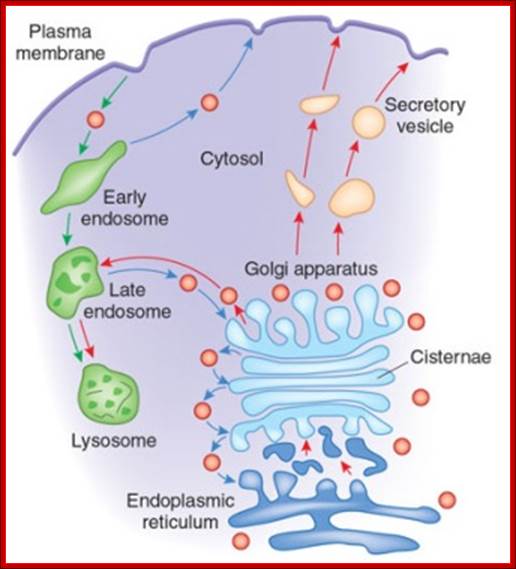
The Golgi apparatus modifies and sorts proteins for transport throughout the cell. The Golgi apparatus is often found in close proximity to the ER in cells. Protein cargo moves from the ER to the Golgi, is modified within the Golgi, and is then sent to various destinations in the cell, including the lysosomes and the cell surface.� 2009 Nature Publishing Group Xu, D. & Esko, J. D. A Golgi-on-a-chip for glycan synthesis. Nature Chemical Biology 5, 612�613 (2009). All rights reserved. The Golgi apparatus transports and modifies proteins in eukaryotic cells. How have scientists studied dynamic protein movements through the Golgi; Pamela L. Connerly, Ph.D.;http://www.nature.com/
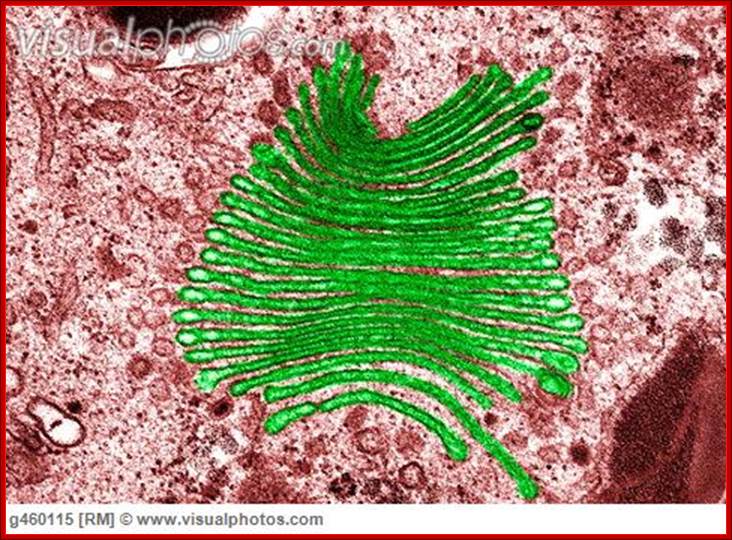
Golgi apparatus colored (TEM); http://www.visualphotos.com/
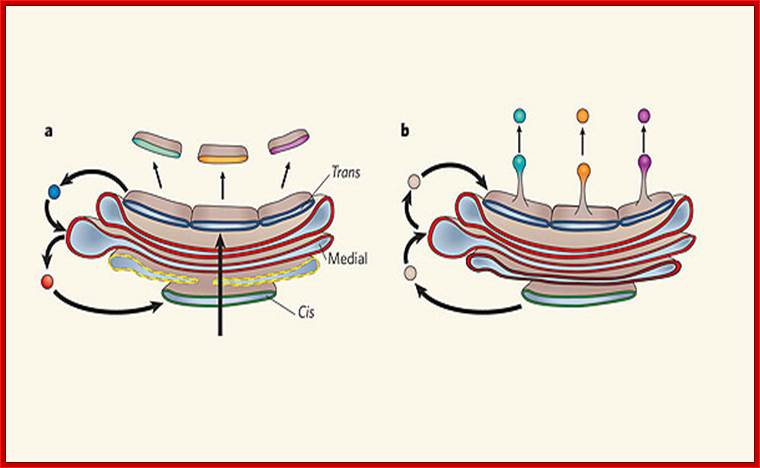
Two models of protein trafficking through the Golgi
(A) The cisternal maturation model of protein movement through the Golgi. As a new cis cisterna is formed it traverses the Golgi stack, changing as it matures by accumulating medial, then trans enzymes through vesicles that move from later to earlier cisternae (retrograde traffic). (B) The vesicular transport model, where each cisterna remains in one place with unchanging enzymes, and the proteins move forward through the stack via vesicles that move from earlier to later cisternae (anterograde traffic). Malhotra, V. & Mayor, S.;http://www.nature.com/
Intermediate Filaments - Intermediate filaments are a very broad class of fibrous proteins that play an important role as both structural and functional elements of the cytoskeleton. Ranging in size from 8 to 12 nanometres�, intermediate filaments function as tension-bearing elements to help maintain cell shape and rigidity.
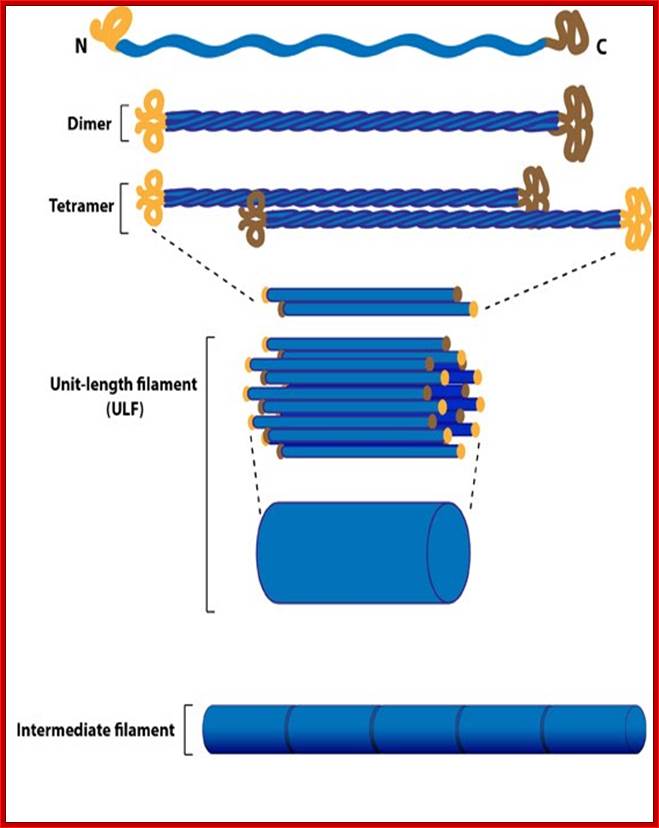
Intermediate Filament Assembly: IFs are heterogenous, there is a greater sequence variations in their genes and produce greater variety of the proteins. Intermediate filaments are built from monomers that associate with each other form dimers. Pairs of dimers then associate in an anti-parallel fashion to form staggered tetramers. Lateral associations between eight tetramers form unit-length filaments, which are able to anneal to each other, end-to-end, to form intermediate filaments. TypeI and II: Keratins; Type III; Desmin and Vimentins; Type IV; Neurofilaments; and Type V; Lamins; http://static.mechanobio.info/Home/list-of-figures/image-cabinet/intermediate_filaments.jpg
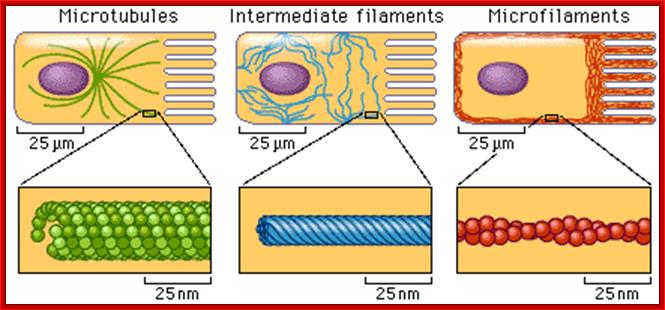
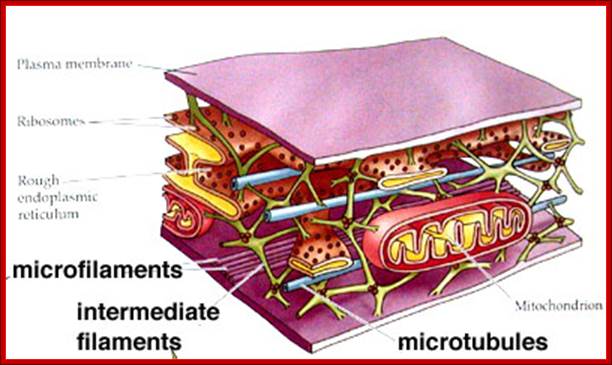
The cytoskeleton consists of microtubules,
intermediate fibers, and microfilaments, which together maintain cell shape,
anchor organelles, and cause cell movement. The microtubules and microfilaments
are frequently assembled and disassembled according to cellular needs for
movement and maintaining cell shape. Intermediate filaments are more permanent
than microtubules and microfilaments.
The cell diagrams shown here represent
intestinal epithelial cells with finger-like projections, the microvilli. The
location and appearance of cytoskeletal fibers in different cell types will
vary. http://www.phschool.com/
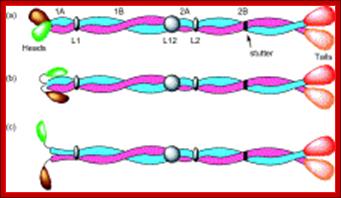
Top Fig.: Schematic diagram of (a) an intermediate filament heterodimer with coiled-coil domains 1A, 1B, 2A, and 2B, and noncoiled-coil connecting linkers L1, L12, and L2. A stutter occurs in the heptad substructure at a point close to the center of segment 2B. The N-terminal �globular� domains (green for Type I and brown for Type II chains) are termed the heads, and the C-terminal domains (red for Type I and orange for Type II chains) are designated the tails. In (b), the heads are shown folded back over the rod domain, where it is believed that this will stabilize segment 1A. In (c), the heads are shown away from the body of the rod domain and in a position where they can interact more easily with other cellular entities. As a consequence, segment 1A may become destabilized and hence unwind to form two separate α-helical strands.
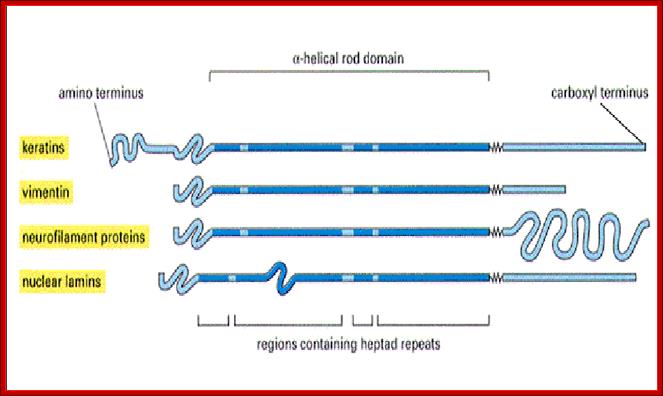
Middle Fig; Each filament monomer consists of an alpha helical rod domain which connects the amino acid head and carboxyl tail� The figure above is from Mol.Biol of the cells from Alberts et al
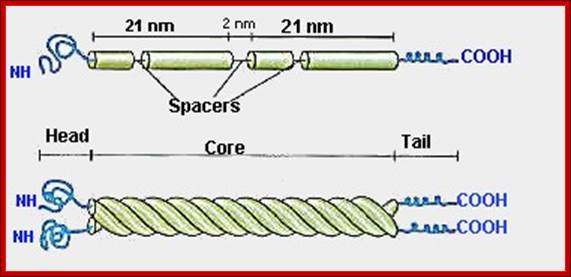
Bottom fig; Formation of protofilaments; The rods coil around another filament like a rope to form a dimer.� The and C terminals of each filament are aligned.� Some IF form heterodimers and others form homodimers.� These then form tetramers that line up head to tail. This tetramer is considered the basic subunits if IFs. http://www.cytochemistry.net/
Lysosomes - The main function of these micro bodies is digestion. Lysosomes break down cellular waste products and debris from outside the cell into simple compounds, which are transferred to the cytoplasm as new cell-building materials.
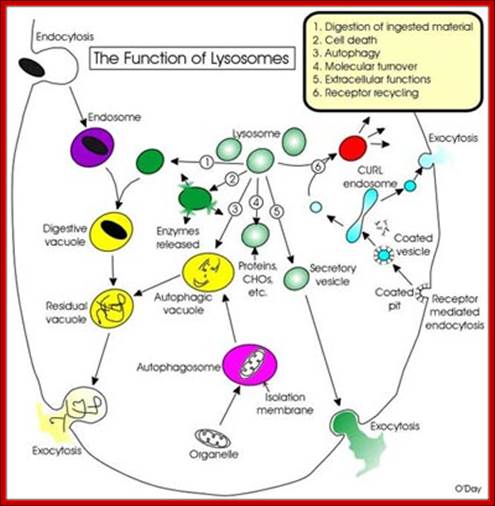
Notes.blogspot.com
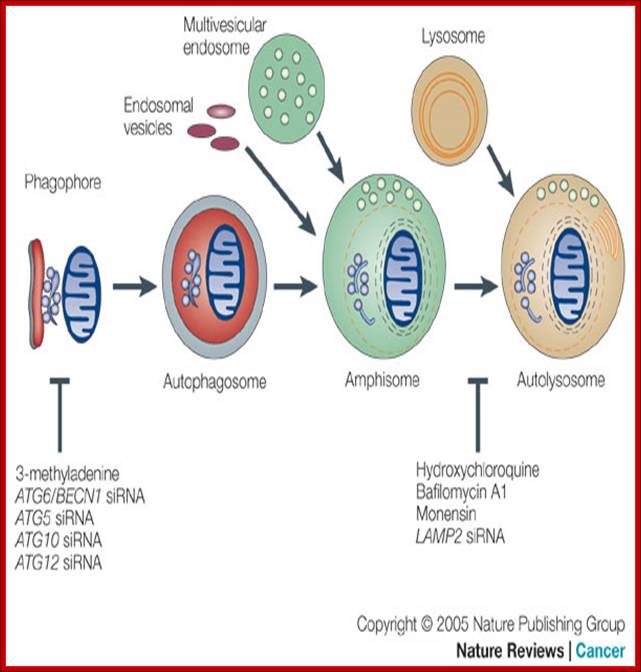
Note that a similar process of sequestration occurs in micro-autophagy. However, it is the lysosomal membrane itself that deforms to engulf the cytosolic substrate in this case. In a third form of autophagy, chaperone-mediated autophagy, soluble cytosolic proteins bind to cytosolic chaperones, forming complexes that translocate into the lysosomal lumen through a lysosomal membrane receptor; Guido Kroemer & Marja J��ttel�
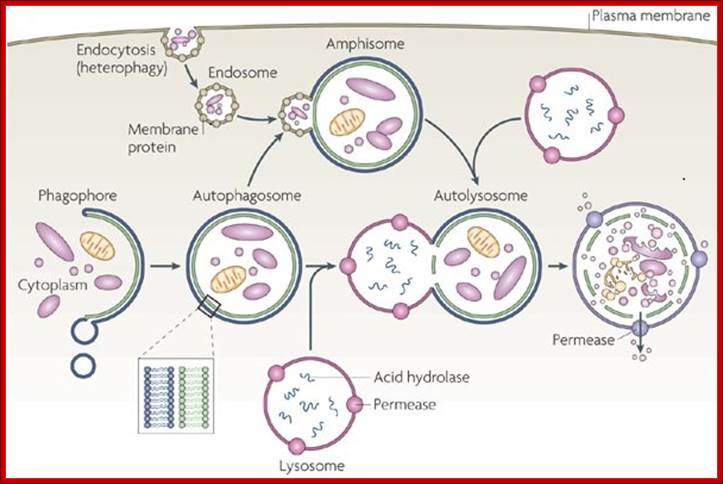
The formation of phagolysosomes.
During autophagy, sequestration begins with the formation of a phagophore that expands into a double-membrane autophagosome while surrounding a portion of the cytoplasm. The autophagosome may fuse with an endosome (the product of endocytosis), which is a form of heterophagy (Heterophagy occurs when the cell internalizes and degrades material that originates outside of the cell. In contrast, autophagy occurs when the cell consumes part of itself). The product of the endosome-autophagosome fusion is called an amphisome. The completed autophagosome or amphisome fuses with a lysosome, which supplies acid hydrolases[KG1] . The enzymes in the resulting compartment, an autolysosome, break down the inner membrane from the autophagosome and degrade the cargo. The resulting macromolecules are released and recycled in the cytosol. Susana Castro-Obregon;http://www.nature.com/
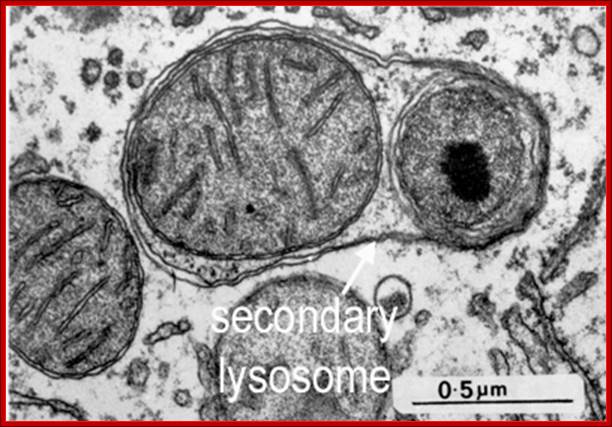
Electron micrograph showing four dark secondary lysosomes surrounded by numerous mitochondria; De Histology; http://histology.leeds.ac.uk
Lysosomes are important for breaking down proteins. There are several 'lysosomal' storage diseases, where a mutation in one of the lysosomal enzymes means that it does not work properly, and proteins can accumulate in lysosomes as they cannot be digested. An example is Hurler's disease, in which an enzyme which breaks down glycosoaminoglycans is missing, and lysosomes accumulate in massive quantities. Lysosomes are important for breaking down proteins. There are several 'lysosomal' storage diseases, where a mutation in one of the lysosomal enzymes means that it does not work properly, and proteins can accumulate in lysosomes as they cannot be digested. An example is Hurler's disease, in which an enzyme which breaks down glycosoaminoglycans is missing, and lysosomes accumulate in massive quantities. http://www.histology.leeds.ac.uk/
This process is diagrammed in the cartoon. Mitochondria replicate much like bacterial cells. When they get too large, they undergo fission. This involves a furrowing of the inner and then the outer membrane as if someone was pinching the mitochondrion. Then the two daughter mitochondria split. Of course, the mitochondria must first replicate their DNA. This will be discussed in more detail in the next section. An electron micrograph depicting the furrowing process is shown in these figures. The figure on the right was taken from Fawcett, A Textbook of Histology, Chapman and Hall, 12th edition, 1994;� http://www.cytochemistry.net/
Proteolysis: from the lysosome to ubiquitin and the proteasome: Aaron Ciechanover
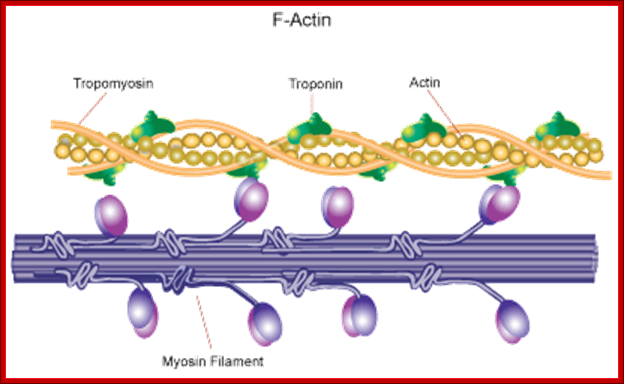
� Microfilaments - Microfilaments are solid rods made of globular proteins called actin. These filaments are primarily structural in function and are an important component of the cytoskeleton.
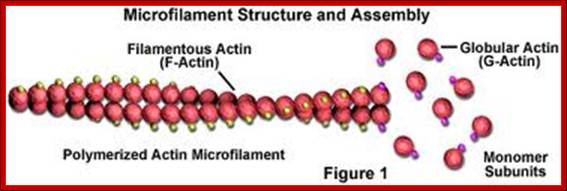
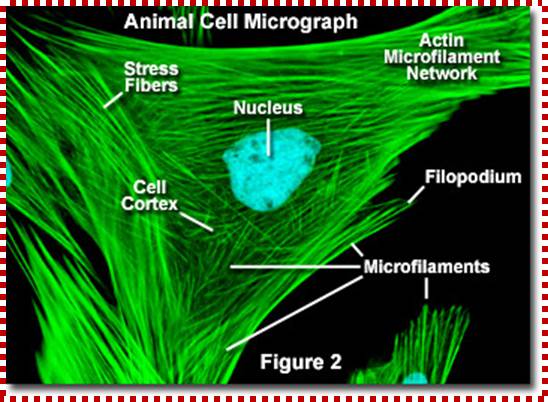
http://micro.magnet.fsu.edu/
Microfilaments: http://envorganelles.wikispaces.com/
http://www.irarosenberg.com/
Sources and All That Jazz:
Epiehonorsbiology-
http://www.biology.arizona.edu/cell_bio/tutorials/cytoskeleton/page1.html
http://micro.magnet.fsu.edu/cells/microfilaments/microfilaments.html
����������������������������������������������� Sigma-Aldrich
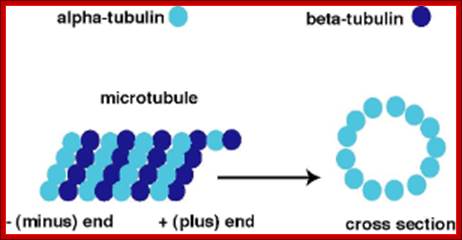
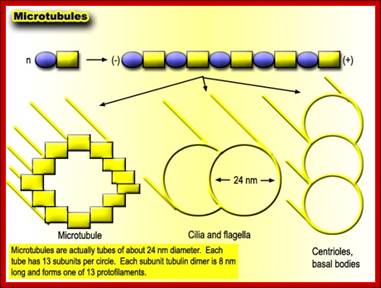
� Microtubules - These straight, hollow cylinders are found throughout the cytoplasm of all eukaryotic cells (prokaryotes don't have them) and carry out a variety of functions, ranging from transport to structural support.
Microtubules.
The microtubule is a hollow cylinder composed of
11-15 stands of tubulin dimers (alpha-tubulin and beta-tubulin). The plus end
of the microtubule has an exposed beta-tubulin
and is typically the growing end of the microtubule. The minus end has an
exposed alpha-tubulin
and is typically the shrinking end of the microtubulehttps://wikispaces.psu.edu
http://classes.kumc.edu/
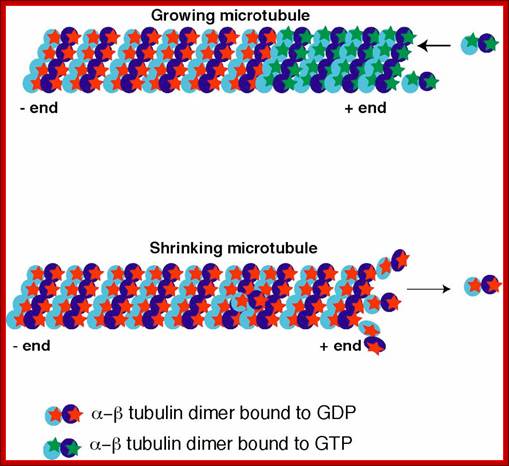
Dynamic instability
The plus end of the microtubules grows by the
addition of tubulin dimmers bound to GTP. With time the GTP is hydrolyzed to
GDP. Normally the rate of polymerization at the plus end is more rapid than the
rate of GTP hydrolysis so the plus end maintains a GTP cap (tubulin-GTP dimer).
If the rate of GTP hydrolysis exceeds the rate of polymerization the GTP cap is
lost and the plus end undergoes rapid depolymerization. https://wikispaces.psu.edu
Microtubules come in three flavours: single, double, and triple. Single microtubules have thirteen "protofilamentss", i.e. single chains of tubulin dimers, in their circumference. In cilia and flagella, double microtubules exist, with the second tubule using three protofilamentss of the primary, and ten additional protofilamentss. Logic suggests that these double microtubules would be more rigid than the single ones, but the ciliary structure nevertheless is constructed to be motile. Finally, triple microtubules form the structure of centrioles, as shown in the inset of the figure below.;http://classes.kumc.edu/J. Victor Small, et al
Mitochondria - Mitochondria are oblong shaped organelles that are found in the cytoplasm of every eukaryotic cell. In the animal cell, they are the main power generators, converting oxygen and nutrients into energy.
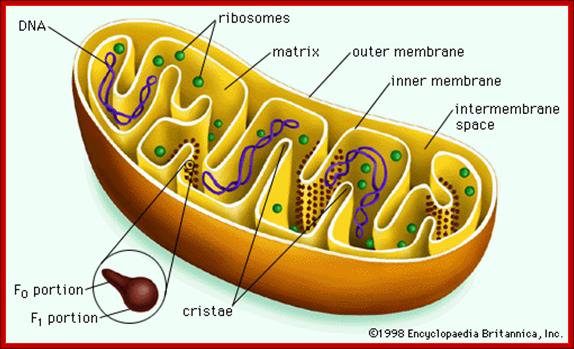
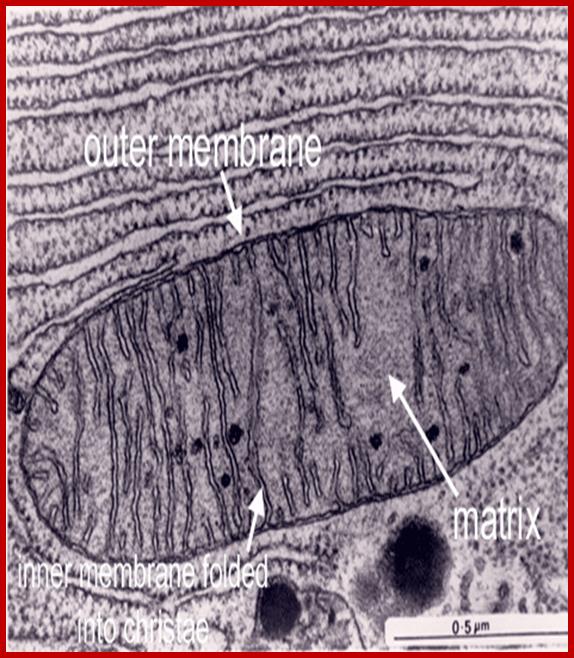
The Mitochondria is the cell organelle that releases energy from stored food molecules. It uses energy from food to make high energy compounds that the cell can use to power growth, development and movement. They are enclosed to envelope membranes. The inner membrane is folded. http://www.mthira.vic.edu.au/
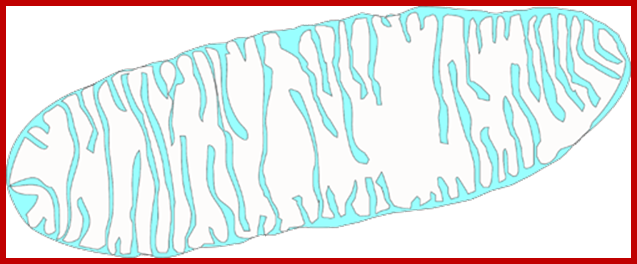
����������������������������������� http://histology.leeds.ac.uk
TEM picture; http://blog.coturnix.org/
Mitochondrion is surrounded by ER, Inner structural features, Cristae, Matrix
The build-up of the ER�mitochondria junctions; Close interactions between the endoplasmic reticulum (ER) and mitochondria are essential for rapid and sustained Ca2+ uptake by mitochondria. Voltage-dependent anion channels (VDACs), located at the outer mitochondrial membrane (OMM), are responsible for the rapid transfer of Ca2+ from the ER�mitochondria apposition, and their function results in high Ca2+ microdomains in the mitochondria intermembrane space. Accumulation of Ca2+ into the mitochondrial matrix occurs via the mitochondrial Ca2+ uniporter (MCU), which rapidly accumulates Ca2+ across the steep electrochemical gradient. A number of chaperones and regulatory proteins control the formation of the ER�mitochondria junction, the clustering of signalling proteins and their modulation. Mitofusin 2 (MFN2) is involved in both mitochondrial fusion and in ER�mitochondria tethering, by both homotypic interactions and heterotypic interactions with MFN1. Chaperones modulate ER Ca2+ buffering (for example, calreticulin and calnexin) and control the stability or the sorting of signalling proteins. For example, sigma 1 receptor stabilizes inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) receptors (Ins(1,4,5)P3Rs) when ER Ca2+ stores are depleted, thereby ensuring proper Ca2+ fluxes from the ER to the mitochondria. Phosphofurin acidic cluster sorting protein 2 (PACS2) controls the translocation of calnexin from the ER to the plasma membrane and thereby modulates ER Ca2+ buffering and controls ER�mitochondria appositions during apoptosis. Moreover, chaperones affect the activity of ion channels. For example GRP75 (75 kDa glucose-regulated protein), which mediates the interaction of VDAC1 with Ins(1,4,5)P3R, facilitates mitochondrial Ca2+ uptake, and PML (promyelocytic leukaemia) protein, which regulates Ins(1,4,5)P3R-mediated Ca2+ release from the ER, supports mitochondrial Ca2+ uptake and thus has a crucial role during apoptosis. The family of long-chain fatty-acid CoA ligases (FACL) is involved in lipid metabolism and is enriched in mitochondria-associated membranes (MAMs). IMM, inner mitochondrial membrane. Rosario Rizzuto, Diego De Stefani, Anna Raffaello & Cristina Mammucarihttp://www.nature.com/
The mitochondrion has four compartments: an outer membrane, an inner membrane (made of cardiolipin), an intermembrane space (between outer and inner membranes), and a matrix (inside inner membrane). The processes that happen in the mitochondrion are pyruvate oxidation, the Krebs cycle, the metabolism of amino acids, fatty acids, and steroids, and generation of adenosine triphosphate (ATP). ATP, which is used for energy, is made through the electron-transport chain and the oxidative-phosphorylation system (respiratory chain) in the inner mitochondrial membrane. (WUSTL)
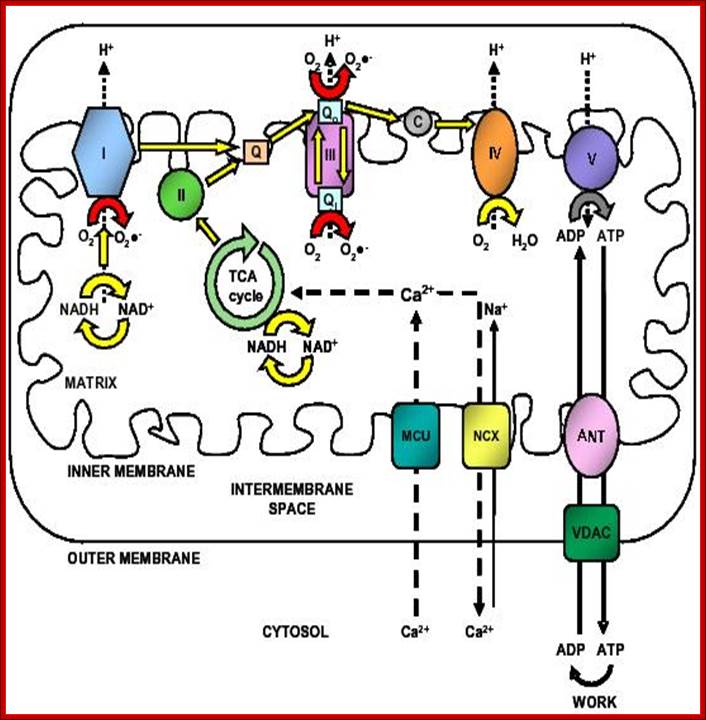
Figure 2. Calcium and mitochondrial function. Calcium entry into the mitochondrial matrix via the mitochondrial calcium uniporter (MCU) triggers activation of the tricarboxylic acid (TCA) cycle, resulting in increased NADH production. NADH triggers the movement of electrons down complexes (I-V) of the electron transport chain (ETC) by initially donating electrons to complex I. These electrons are then transferred to coenzyme Q (Q). Complex II uses the conversion of succinate to fumarate (produced by the TCA cycle) to also transfer electrons to coenzyme Q. Electrons at coenzyme Q are transferred to complex III. These electrons are then transferred to complex IV via cytochrome c (C). Complex IV is the terminal electron accepter which acts to convert O2 to water. Complexes I, III and IV pump protons (H+) from the matrix into the intermembrane space. This creates a proton motive force which is used by complex V to convert ADP into ATP. ATP is released into the cytosol via the adenine nucleotide transporter (ANT) and the voltage-dependent anion channel (VDAC) where it is subsequently converted to ADP during ATP-dependent processes (Work). ADP then re-enters the mitochondrial matrix. Some electrons passing through the ETC leak into either the matrix or intermembrane space where they react with oxygen to form superoxide (O2−�). Complex I releases superoxide towards the matrix. Complex III releases superoxide toward both the matrix and the intermembrane space via Qi and Qo respectively.62,63Calcium is extruded by the mitochondria via the Na+/Ca2+ exchanger. Dashed arrows indicate movement of Ca2+. Electron flow is indicated by yellow and red arrows. Dotted arrows indicate movement of H+. Solid lines represent movement of ADP and ATP. Red arrows indicate electron flow involved in the production of Ross-Helena M. Viola and Livia C. Hool https://encrypted-tbn3.gstatic.com/images; http://aups.org.au
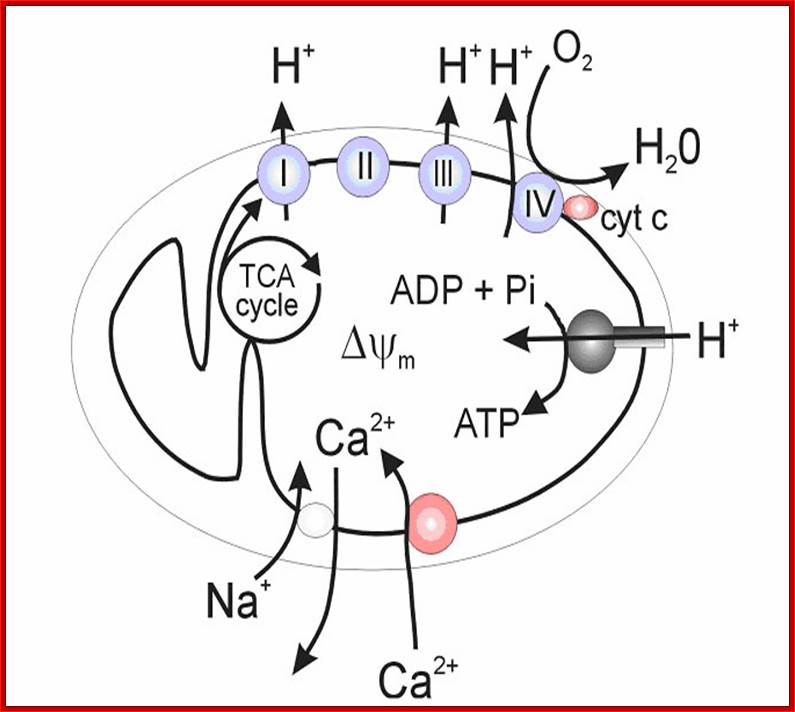
https://encrypted-tbn3.gstatic.com/images; http://aups.org.au
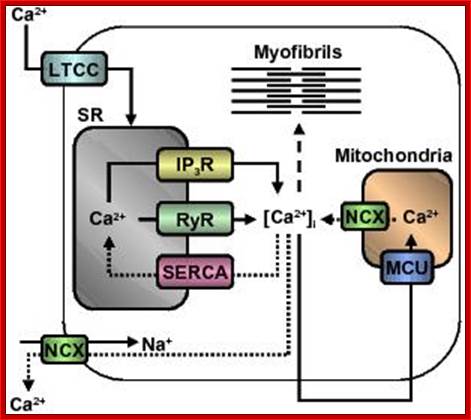
Figure. Calcium regulation in the heart; Calcium channels and transporters involved in initiating contraction (solid arrows) by calcium-induced calcium release mechanism and subsequent relaxation (dotted arrows) in myofibres. Dashed line indicates calcium activation of myofibrils. Abbreviations: LTCC, L-type Ca2+ channel; SR, sarcoplasmic reticulum; IP3R, inositol triphosphate receptor; RyR, ryanodine receptor; SERCA, sarcoplasmic reticulum Ca2+-ATPase; [Ca2+]i, intracellular calcium concentration; MCU, mitochondrial calcium uniporter; NCX, Na+/Ca2+ exchanger. http://aups.org.au
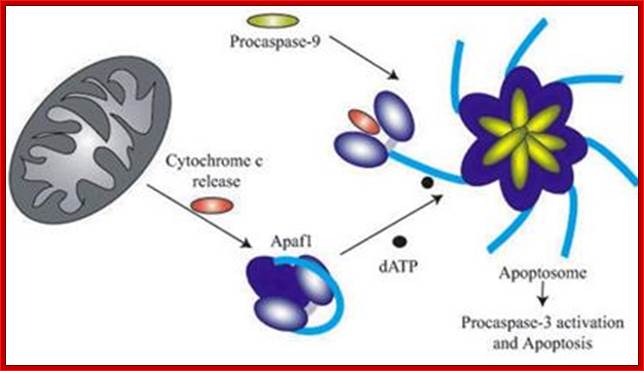
The above diagram depicts interaction of Bid with Bcl2-Bax transmembrane components located in the outer mitochondrial membrane leading to Bax pore formation and the release of Cyt C. The above figure shows the released Cyt C� binds to Apaf-1 at CARD domain , which is further activated by ATP.� This Y� complex binds to procaspase-9 and activates to cleave it and form heptameric complex called Apoptosome, which inturn activates procaspase-3 that leads to devastating effect on cellular components and cell death. The conformational changes in the APAF1 molecules lead to apoptosome formation and to the activation of apoptosis. However, the assembly and the functioning of the apoptosome is regulated by mithocondrial and cytosolic factors (modif. from E. Ferraro et al. 2004); APAF1 is the structural core of the apoptosome. When the mitochondrial pathway of apoptosis is activated, cytochrome c is released from mitochondria to cytosol, and then binds to APAF1 CARD domain changing its conformation. A further binding of ATP molecules mediates a second conformational change which leads to open APAF1 conformation. By means of the CARD domain, seven APAF1 molecules bind to each other and to seven molecules of initiator Caspase-9 forming the apoptosome and causing the activation of effector caspases. The formation of apoptosome and the activation of caspases are regulated by numerous interacting proteins. CED-4 (C. elegans); DARK (D. melanogaster); CARD proteins. http://atlasgeneticsoncology.org/
Mitochondrial-DNA. https://encrypted-tbn3.gstatic.com/images
� Nucleus - The nucleus is a highly specialized organelle that serves as the information processing and administrative centre of the cell. This organelle has two major functions: it stores the cell's hereditary material or DNA, and it coordinates the cell's activities, which include growth, intermediary metabolism, protein synthesis, and reproduction (cell division).
Normal somatic cells have highly compartmentalized nuclear structure;
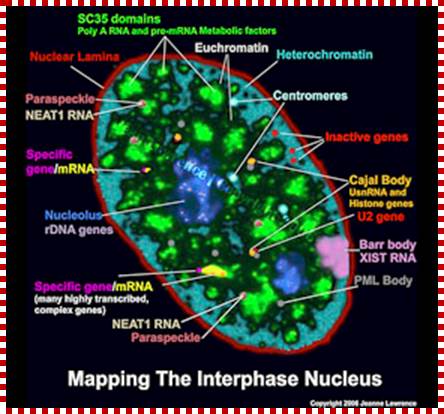
Neat RNA is an architectural RNA that scaffolds a large nuclear structure; Neat RNA is a non-coding RNA that is required for the formation of paraspeckles (see image below). Paraspeckles are ubiquitous nuclear structures (~10-30/nucleus) of unknown function found in all human primary and transformed cells. https://www.umassmed.edu/
https://i.ytimg.com
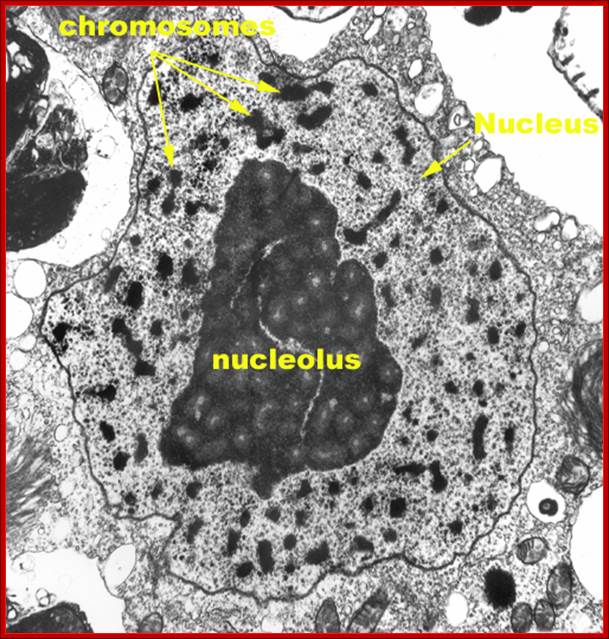
http://triemerlab.plantbiology.msu.edu/
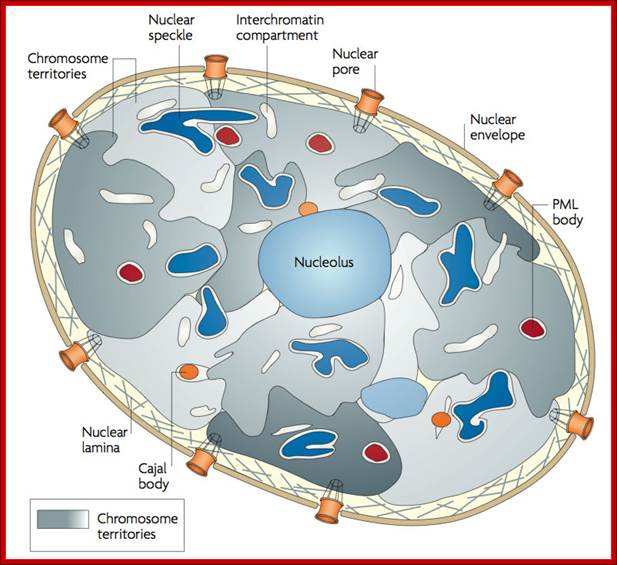
������������������������������������������ ������������� http://cellbiology.med.unsw.edu.au/
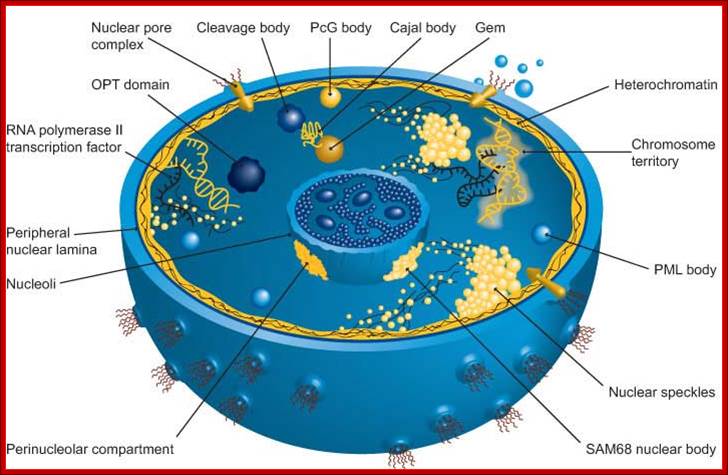
The nucleus is separated from the cytoplasm by a double membrane. The outer nuclear membrane is continuous with the endoplasmic reticulum (Spector 2001; Lamond and Sleeman 2003). Exchange of proteins and mRNA between the cytoplasm and thenucleus occurs through multi-protein structures situated in the nuclear envelope known as nuclear pores. The nucleus is compartmentalized and contains numerous sub-nuclear bodies, including nucleoli, splicing speckles, Cajal bodies (CB), gems, andpromyelocytic leukaemia (PML) bodies in addition to chromosomes. In contrast to cytoplasmic compartments, the sub-nuclear bodies lack a membrane separating them from the nucleoplasm. The build-up of factors in these distinct sub-nuclear bodies may serve to enhance the efficiency of specific nuclear processes. http://www.abcam.com/
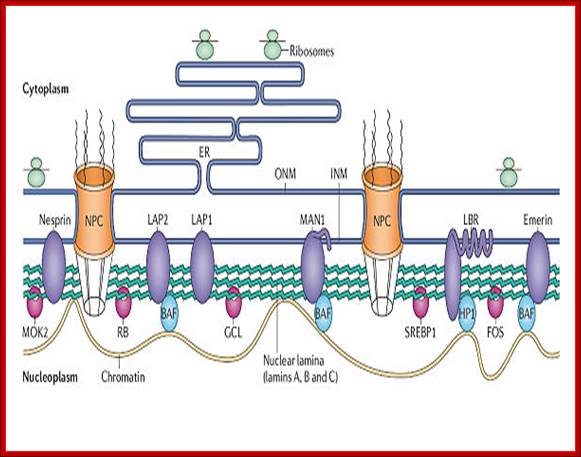
Structure and function of the nuclear lamina. The nuclear lamina lies on the inner surface of the inner nuclear membrane (INM), where it serves to maintain nuclear stability, organize chromatin and bind nuclear pore complexes (NPCs) and a steadily growing list of nuclear envelope proteins (purple) and transcription factors (pink). Nuclear envelope proteins that are bound to the lamina include nesprin, emerin, lamina-associated proteins 1 and 2 (LAP1 and LAP2), the lamin B receptor (LBR) and MAN1. Transcription factors that bind to the lamina include the retinoblastoma transcriptional regulator (RB), germ cell-less (GCL), sterol response element binding protein (SREBP1), FOS and MOK2. Barrier to autointegration factor (BAF) is a chromatin-associated protein that also binds to the nuclear lamina and several of the aforementioned nuclear envelope proteins. Heterochromatin protein 1 (HP1) binds both chromatin and the LBR. ONM, outer nuclear membrane. Coutinho et al. Immunity & Ageing 2009; http://en.wikipedia.org/
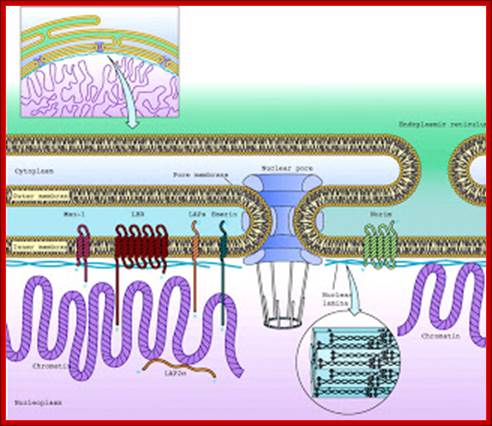
Nuclear lamina
Nuclear lamina is a fibrous meshwork that provides structural support to the nucleus. It is composed of lamina which is 60-80kd fibrous protein. Mammals has basically 3 lamin genes A, B and C which codes for atleast 7 distinct proteins. Laminal protein is polar in nature. At the first stage of its development its subunit unites to form dimer and than α-helical region wound around each other to form a structure called coiled coil, now these associate with each other to form nuclear lamina. The association of lamins with nuclear membrane is facilitated by the posttranslation addition of lipid in particular prenylation of c-terminal cysteine residue. In addition, the lamins bind to specific inner nuclear membrane protein such as emerin and the laminB receptor, mediating their attachment to the nuclear envelope and lacalizing and organizing them within the nucleus. Nuclear lamina also binds to chromatin through histones H2A and H2B as well as other chromatin proteins while binds to DNA directly; http://biotechhelpline16.blogspot.com/
A schematic view of inner nuclear membrane proteins and their binding interactions with the nuclear lamina and nucleoplasmic components.
The outer and inner nuclear membranes (ONM and INM, respectively) are shown in cross-section, with a nuclear pore complex spanning the two membranes. The exact interactions and organisation of the inner nuclear membrane, nuclear lamina and chromatin are unknown and are hypothetically depicted in this figure. Twelve inner nuclear membrane proteins have been characterised in the mammalian nuclear envelope. These include: the multi-spanning membrane proteins nurim, lamin B receptor (LBR), ring-finger-binding protein (RFBP); the double-spanning membrane protein MAN1; and the single-spanning membrane proteins emerin, lamina-associated protein 2 (LAP-2) isoforms (b, g, d, e) and LAP-1 isoforms (A, B, C). All the LAP-2 isoforms, emerin and MAN1 share a homologous N-terminal domain called the LEM domain, which binds to BAF (�barrier to auto-integration factor�). Interactions occur between the inner nuclear membrane proteins and the A-type lamins (shown in blue) and B-type lamins (shown in orange), which are helical filamentous proteins of the nuclear lamina and nucleoplasm. Intranuclear lamins bind to the soluble LAP-2 isoform LAP-2a. Transcriptional regulators crosslink inner nuclear membrane proteins and chromatin. These include: the retinoblastoma protein pRB; the �germ-cell-less� protein GCL; the transcription factor E2F; and RNA polymerase, RNA splicing complex and DP protein. Heterochromatin-binding proteins include HP1, BAF and HA95 (fig002jel).
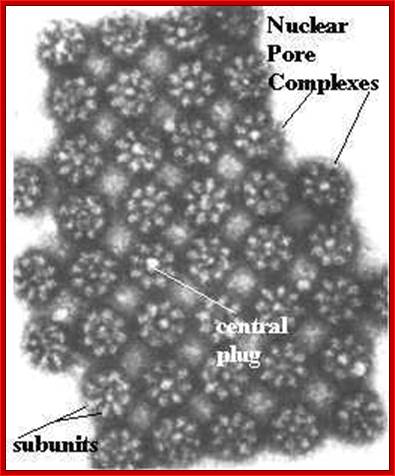
Nuclear pore complex; http://biologica.concord.org/
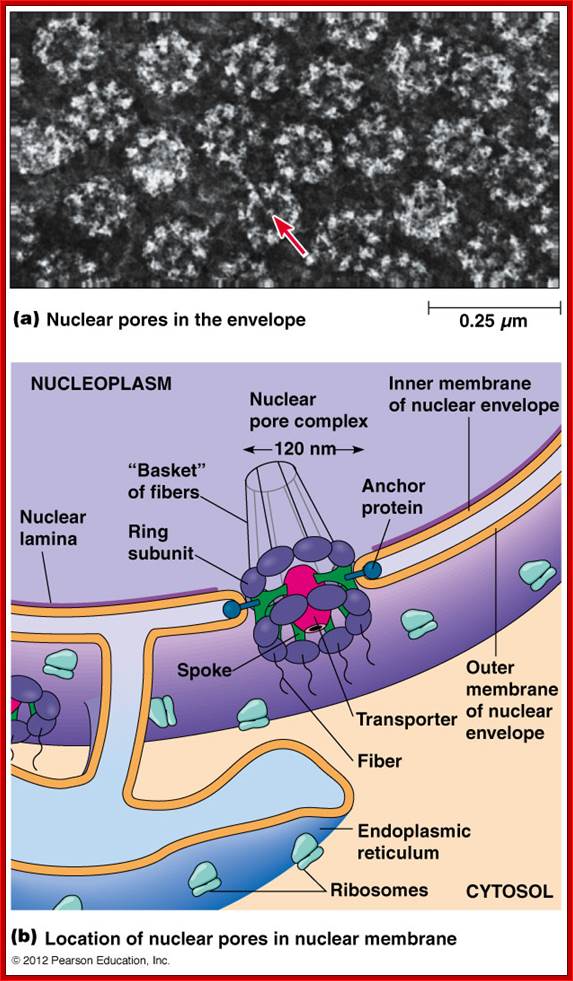
A double-membrane nuclear envelope surrounds the nucleus. The inner and outer nuclear membranes
(7-8 nm thick) are surrounded by the periplasmic space of 20-40 nm thickness.
The inner nuclear membrane is supported by the
nuclear lamina fibres. The outer membrane is continuous with the endoplasmic reticulum and
ribosomes often on the cytosolic side.
The periplasmic space is continuous with the
ER's lumen. Nuclear pores are
channels that pass through both nuclear membranes that join the cytosol with
the nucleoplasm.
The inner and outer membranes fuse to form a
channel that is lined with the nuclear pore complex (diameter 120 nm). A mammalian nucleus may have 3000-4000
nuclear pores.
Nuclear pores are site of molecular entry and exit both the
passive transport of small molecules and the active transport of large proteins
and RNA. Proteins that are
required in the nucleus contain nuclear localization sequences (NLS) that
target them through the nuclear pores.
The NLS-containing proteins bind importin (a
cytosolic receptor protein) which binds the protein to the nuclear pore. The nuclear pore transporter then
passes the protein into the nucleoplasm. http://www.mun.ca/
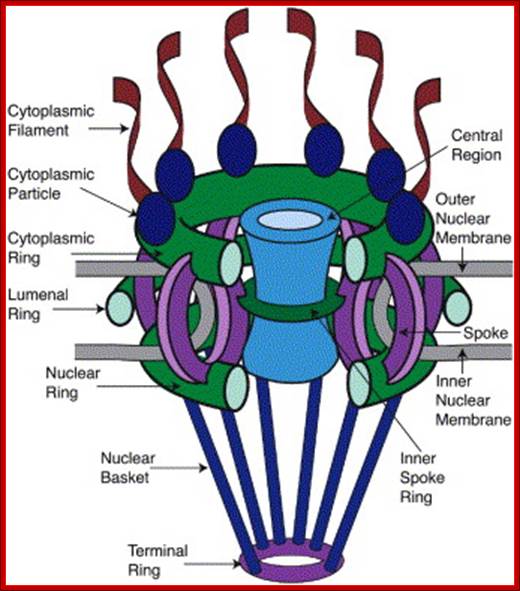
Figure. Schematic Diagram of NPC SubstructuresThe structure has an apparent 8-fold rotational symmetry perpendicular to the plane of the nuclear membrane; however, certain facing portions are removed in this diagram to reveal the architecture of the central region. Labels for all the structures found in vertebrate NPCs are included, with the cytoplasmic face on top. Adapted from the model in Rout and Wente, 1994.
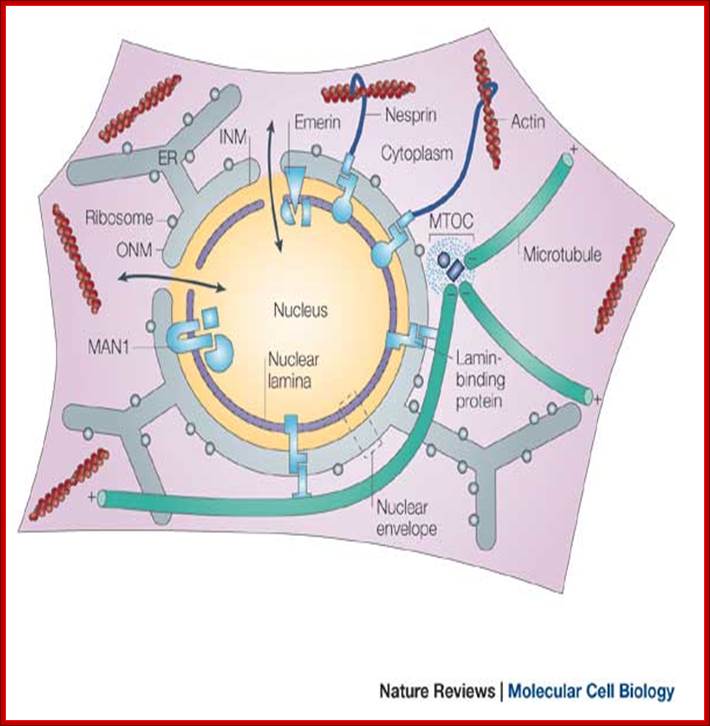
The nuclear envelope (NE) has two membranes (inner and outer; INM and ONM, respectively), which join to form 'pores' that are occupied by nuclear pore complexes (NPCs; not depicted). For clarity, only two nuclear pores are depicted. NPCs mediate traffic in and out of the nucleus, as indicated by the double-headed arrows. The NE is continuous with the endoplasmic reticulum (ER) network. Microtubules, which originate at the centrosome (or microtubule organizing center; MTOC), and microtubule-dependent motors (not shown) regulate the position of the nucleus by attaching to lamin-dependent protein complexes (shown as blue interlocking shapes) at the NE. Certain ONM-localized members of the nesprin protein family attach to actin filaments (red). The proposed nuclear actin network (to which emerin and A-type lamins bind) is depicted in . The lamin-filament network near the INM is shown in purple; internal lamins are not shown. Lamin-dependent complexes that are formed by integral INM proteins, such as emerin (blue triangle) and MAN1 (blue U-shape) are shown in , respectively. In normal cells, proteins that localize specifically to the INM (or ONM) give bright fluorescent signals at the NE when they are stained by indirect immunofluorescence (not depicted). In cells that lack A-type lamins (not depicted), many of these proteins are not retained at the NE but instead drift throughout the NE/ER network. Yosef Gruenbaum, Ayelet Margalit, Robert D. Goldman, Dale K. Shumaker & Katherine L. Wilson http://nature.com.
Schematic diagram of NE dynamic organization and its relationship to euchromatin and heterochromatin. The INM and the ONM of the NE are joined at the nuclear pore complexes (NPCs). Integral INM (green) and their associations with chromatin proteins (blue) are indicated. Lamins A/C (black line) and lamin B (red line) are shown as filaments at the nuclear periphery (lamin A/C, lamin B) and in the nucleoplasm (lamin A/C). The binding of the common chromatin binding LEM domain (yellow circles) of several INM proteins and BAF is shown. The bindings of the transcriptional repressors (purple circle); retinoblastoma (Rb) to lamin A/C and GCL to LAP2β and Emerin, are shown. The two chromatin states, of gene-active unwrapped euchromatin at the nucleoplasm (A), and of gene-silenced condensed heterochromatin at the vicinity of the NE (B) are illustrated. In the latter state, the specific bindings of LBR to Heterochromatin Protein 1 (HP1) through histones H3/H4, and of LAP2β to BAF are indicated. Raz Somech, Sigal Shaklai, Ninette Amariglio, Gideon Rechavi and Amos J Simon http://www.nature.com/
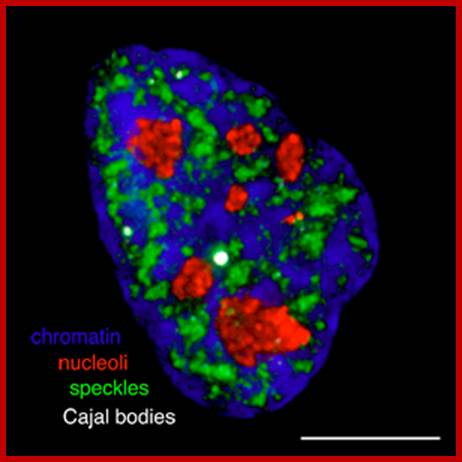
Nucleoli, speckles and cajal bodieshttps://www.st-andrews.ac.uk
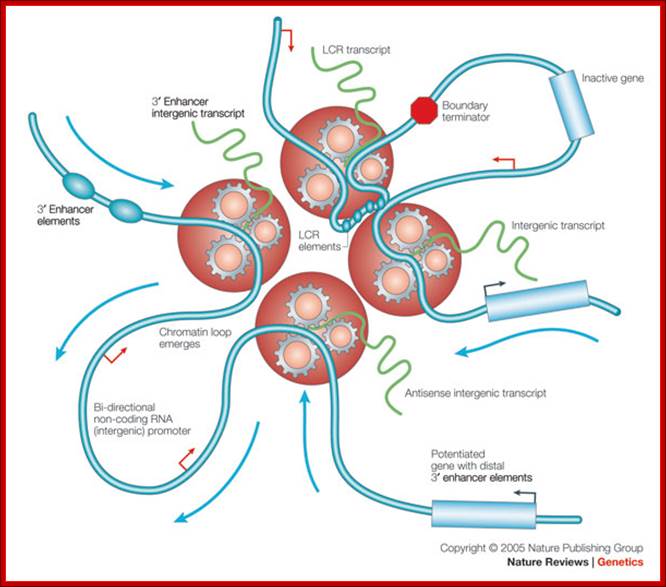
Replication and Transcription. Shaping the landscape of the genome; Clustered RNA polymerase II motor proteins organize genes and distal regulatory elements by using non-coding intergenic transcription. Rather than polymerases sliding along chromatin, chromatin moves through the factory that is powered by the energy released by the hydrolysis of nucleotide triphosphates during elongating RNA synthesis. Black arrows, gene promoters; blue arrows, direction of chromatin movement; LCR, locus control region; red arrows, intergenic promoters. Lyubomira Chakalova et al. http://www.nature.com/
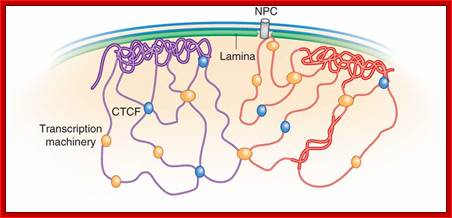
Genomics tools for unraveling chromosome architecture; �Bas van Steensel & Job Dekker; �http://www.nature.com/
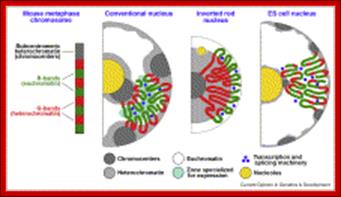
Figure. Models of nuclear architecture and chromosome folding in differentiated and pluripotent cells. In conventional nuclei, zones specialized for expression are often found in between the euchromatic borders of CTs, though transcription factories and speckles are present outside this zone also. In inverted nuclei the euchromatic shells appears to be more homogenous. In ES cells some heterochromatic regions might be decondensed and probabilistically take internal positions; nuclear architecture of pluripotent cells needs further study. Human chromosomes and nuclei differ from the mouse ones primarily by the absence of chromocenters (fused subcentromeric satellite regions). A, B, modified from Solovei et al.
� Peroxisomes - Micro bodies are a diverse group of organelles that are found in the cytoplasm, roughly spherical and bound by a single membrane. There are several types of micro bodies but peroxisomes are the most common.
http://en.wikipedia.org/
� They are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, D-amino acids, polyamines, and biosynthesis of plasminogen�s, i.e. ether phospholipids critical for the normal function of mammalian brains and lungs. A major function of the peroxisome is the breakdown of very long chain fatty acids through beta-oxidation. In animal cells, the very long fatty acids are converted to medium chain fatty acids, which are subsequently shuttled to mitochondria where they are eventually broken down to carbon dioxide and water. In yeast and plant cells, this process is exclusive for the Peroxisomes. Peroxisomes can be derived from the endoplasmic reticulum and replicate by fission. Peroxisomes can be derived from the endoplasmic reticulum and replicate by fission. Peroxisome matrix proteins are translated in the cytoplasm prior to import. Specific amino acid sequences (PTS or peroxisomal targeting signal) at the C-terminus (PTS1) or N-terminus (PTS2) of peroxisomal matrix proteins signals them to be imported into the organelle. There are at least 32 known peroxisomal proteins, called peroxins,
� Plasma Membrane - All living cells have a plasma membrane that encloses their contents. In prokaryotes, the membrane is the inner layer of protection surrounded by a rigid cell wall. Eukaryotic animal cells have only the membrane to contain and protect their contents. These membranes also regulate the passage of molecules in and out of the cells.�
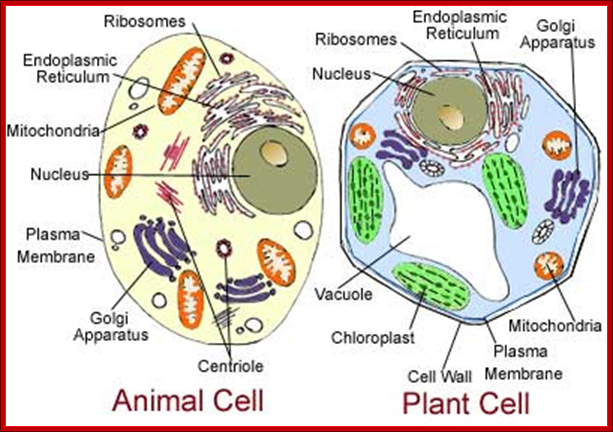
http://leavingbio.net
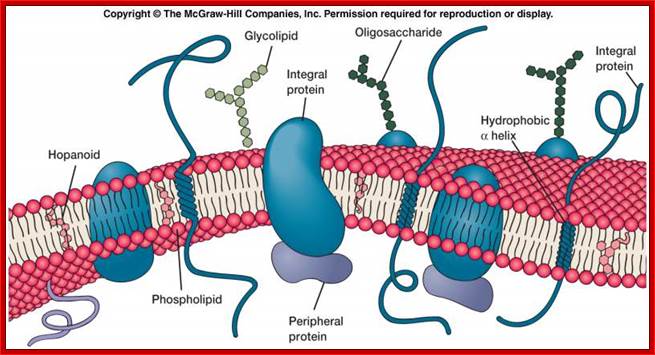
http://classroom.sdmesa.edu/
Structurally PM is made up of bilayer of lipids embedded with a host proteins of different structures and different functions; no living organism can live without PM which separate external environment from its internal microcosm, but communicates.� It is an extraordinary structure.
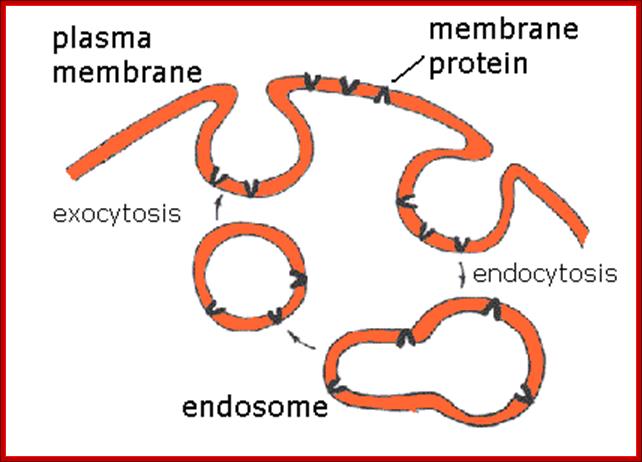
http://courses.washington.edu/
PM has the ability to perform functions like exocytosis and endocytosis.
![]() �
�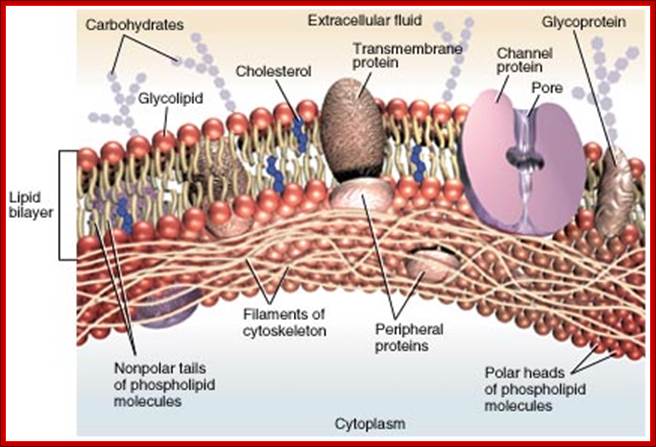
The role of mechanical forces in the regulation of glomerulotubular balance in the proximal tubule (PT) and Ca2+ signaling in the distal nephron was first recognized a decade ago, when it was proposed that the microvilli in the PT and the primary cilium in the cortical collecting duct (CCD) acted as sensors of local tubular flow. In this review, we present a summary of the theoretical models and experiments that have been conducted to elucidate the structure and function of these unique apical structures in the modulation of Na+, HCO3−, and water reabsorption in the PT and Ca2+ signaling in the CCD. We also contrast the mechanotransduction mechanisms in renal epithelium with those in other cells in which fluid shear stresses have been recognized to play a key role in initiating intracellular signaling, most notably endothelial cells, hair cells in the inner ear, and bone cells. In each case, small hydrodynamic forces need to be greatly amplified before they can be sensed by the cell's intracellular cytoskeleton to enable the cell to regulate its membrane transporters or stretch-activated ion channels in maintaining homeostasis in response to changing flow conditions.;ttp://ajprenal.physiology.orghttp://ajprenal.physiology.org
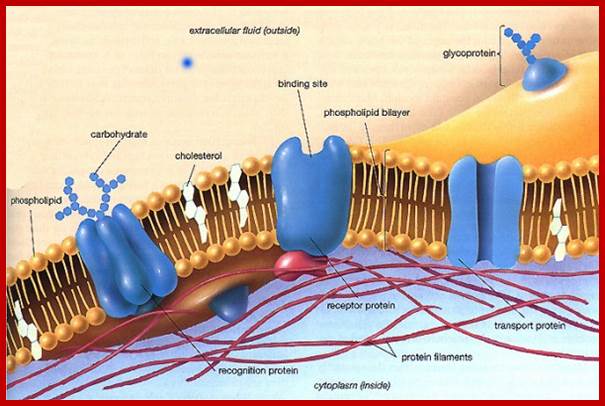
Fluid mosaic model; https://www.desktopclass.com
The plasma (cell) membrane is the boundary between the extracellular fluid and the intracellular fluid. It is composed primarily of phospholipids. The phospholipids are arranged in two layers with their hydrophobic fatty acid tails to the interior of the membrane and their hydrophilic polar heads on the inner and outer surfaces. Proteins are another important component of the plasma membrane. There are two types of proteins: integral (trans membrane) proteins that pass through the entire membrane and peripheral proteins that do not protrude into the phospholipid layer but adhere to the intracellular face of the membrane. Some of the phospholipids and integral proteins have carbohydrate chains attached to them forming glycolipids and glycoproteins.
Cholesterol is also found within the membrane of animal cells. The components of the membrane slowly drift past one another thus making the membrane fluid. This theory of the structure of the plasma membrane is known as the fluid-mosaic model; Copyright � The McGraw-Hill Companies, Inc.
� Ribosomes - All living cells contain ribosomes, tiny organelles composed of approximately 60 percent RNA and 40 percent protein. In eukaryotes, ribosomes are made of four strands of RNA. In prokaryotes, they consist of three strands of RNA.� They are the most dynamic molecular machines next only to flagella and cilia.� They are responsible for binding to mRNAs and translate into polypeptide chains.� When ribosomes are bind to ER they transport polypeptide chains into ER.� Bacteria and Archaea contain 70s ribosomes and Eukaryotes contain 80s ribosomes.� Architecturally both have the same pattern and mechanisms.
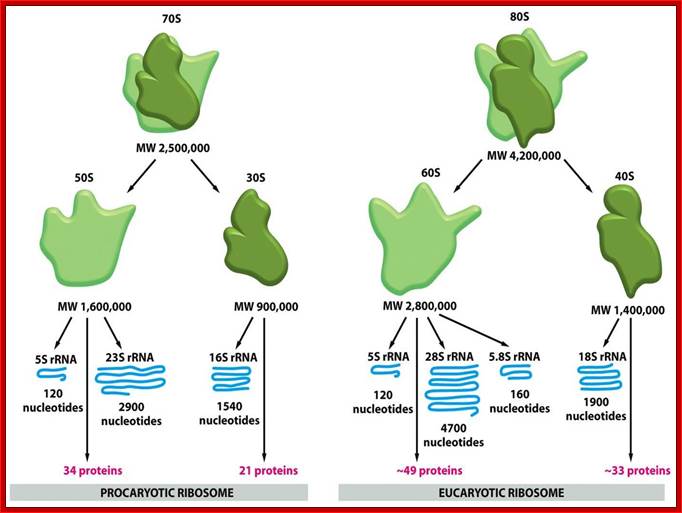
Both are involved in translation of mRNAs; http://www.studyblue.com/
� Glycosomes: They are membrane bound cell organelles contain glycolic enzymes. The membrane is a single unit structure similar to lysosomal membranes.�
Electron micrographic pictures of sections of trypanosomes showing the glycosome ultrastructure. (A) A picture of a bloodstream-form Trypanosoma brucei cell showing the abundance of glycosomes which are predominantly spherical. (B) In procyclic forms, the glycosomes are predominantly elongated organelles. (C) Bloodstream-form T. brucei; many glycosomes can cluster and adopt a very regular organisation. (D) Bloodstream-form T. brucei; many pictures show membrane appendices (indicated by arrows, also in A) facing glycosomes. Bars are 500 nm. G, glycosome; ER, endoplasmic reticulum; FP, flagelar pocket; M, mitochondrion; K, kinetoplast. EM pictures by courtesy of Dr. Isabelle Coppens, Johns Hopkins University, Baltimore, USA;.http://ars.els-cdn.com/content/image/1-s2.0-S0020751911002748-gr1.sml
The glycosomal enzymes are involved in glycogen metabolism.� These are found to be attached to different cell organelles. Functions include glycolysis, purine salvage, beta oxidation of fatty acid and lipid synthesis.
In addition to the optical and electron microscope, scientists are able to use a number of other techniques to probe the mysteries of the animal cell. Cells can be disassembled by chemical methods and their individual organelles and macromolecules isolated for study. The process of cell fractionation enables the scientist to prepare specific components, the mitochondria for example, in large quantities for investigations of their composition and functions. Using this approach, cell biologists have been able to assign various functions to specific locations within the cell. However, the era of fluorescent proteins has brought microscopy to the forefront of biology by enabling scientists to target living cells with highly localized probes for studies that don't interfere with the delicate balance of life processes. It is not only animal cells are used for this kind of studies; even plant cells extensively used for they more structural features which are not found in animal systems.
2. Cells and Tissues of Immune system:
Lymphoid tissues are considered as generative primary lymphoid organs, where lymphocytes (L/C) express antigen receptor and attain phenotype and functional maturity. The peripheral lymphoid cell responds to foreign antigens and initiate responses. Generative lymphoid organs in mammals are bone marrow, where L/C takes their origin from stem cells, which are pleuripotent in nature. Some of them undergo differentiation and develop into B-cells and T-cells. T-cells mature in thymus as functionally competent cells. Lymph nodes, spleen, cutaneous immune system act as peripheral immune system.
Aggregate of L/Cs are also found in connective tissues and virtually in all organs (except central nervous system). Hematopoiesis takes place initially in fetal blood islands of yolk sac, para aortic mesenchyma and in spleen and liver; later this is taken over by bone marrow and increasingly by marrow of flat bones, so that by puberty hematopoiesis occurs mainly in sternum, vertebrae, iliac bone and ribs.
|
Terms |
Definitions.
|
|
|
|
|
|
� 20-40% -CD4+ helper T cells: 50-60% of peripheral blood lymphocytes � -CD8+ cytotoxic T cells: 20-25% of lymphocytes -B cells: � 10-15% of lymphocytes -Natural killer cells: 5-20% of lymphocytes.
|
|
|
|
|
|
�Develop in the bone marrow �Reside in tissues and lymphoid organs �Most derived from� monocyte/macrophage lineage �Phagocytes �Have long projections (dendrites). ��Function in innate immunity responding to microbes �Function in adaptive immunity by transporting antigen from portal of entry to lymphoid organ and presenting antigen to T lymphocytes for activation.
|
|
|
� �Part of innate (natural or native) immunity �5-20% of circulating WBC � �Non-reactivity to self �Kill infected, cancerous or injured cells � �without the need for activation like CD8+T lymphocytes.
|
|
|
� �Early stages of development in bone marrow in mammals � � Immature T cells exit� bone marrow and mature in the � �thymus �Mature CD4+and CD8+T cells exit � thymus & enter periphery; After antigen-specific activation.
|
|
|
� -Secrete cytokines (cytokine pattern distinguishes Th1 vs Th2) � -Provide help to B cells/antibody secretion (Th2: IL-4) � �-Activate macrophages for killing phagocytized organisms (Th1: IFN-γ).
|
|
|
|
|
|
|
|
|
|
|
|
Absorbed interstitial fluid call lymph flows through lymphatic vessels, � �lymph nodes and ultimately into thoracic duct which empties � �into the superior vena cava.
|
|
|
|
|
|
� Enter lymph node via specialized blood vessels called high � endothelial vessels.
|
|
|
|
Types
There are several different types of white blood cells. They all have many things in common, but are all distinct in form and function. A major distinguishing feature of some leukocytes is the presence of granules; white blood cells are often characterized as granulocytes or agranulocytes:
� Granulocytes (polymorph nuclear leukocytes): leukocytes characterized by the presence of differently staining granules in their cytoplasm when viewed under light microscopy. These granules are membrane-bound enzymes that act primarily in the digestion of endocytosed particles. There are three types of granulocytes: neutrophils, basophils, and eosinophils, which are named according to their staining properties.
Granulocytes are
a category of white blood cells characterized by the presence of granulesin
their cytoplasm.[1] They are also called polymorphonuclear leukocytes (PMN, PML, orPMNL) because of
the varying shapes of the nucleus,
which is usually lobed into three segments. This distinguishes them from the
mononuclear agranulocytes.
In common parlance, the term polymorphonuclear
leukocyte often refers
specifically to neutrophil granulocytes,[2] the most abundant of the granulocytes;
the other types (eosinophils, basophils,
and mast cells)
have lower numbers. Granulocytes are produced via granulopoeisis in the bone marrow.
���������������������������������� Granulocytes fight cellular invaders like bacteria and viruses by producing highly reactive oxidants, toxic chemicals that kill microorganisms. Our findings show that in children with severe autism the level of that response was both lower and slower,� said Eleonora Napoli, lead study author and project scientist in the Department of Molecular Biosciences in the UC Davis School of Veterinary Medicine. Neutrophil granulocyte; http://psychcentral.com/
Basophil Granulocyte;https://www.withfriendship.com
�����������������������������������
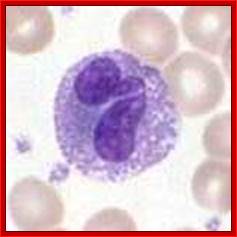
����������������������� Eosinophilic granulocyte; http://www.clker.com
� Agranulocytes (mononuclear leukocytes): leukocytes characterized by the apparent absence of granules in their cytoplasm. Although the name implies a lack of granules these cells do contain non-specific azurophilic granules, which are lysosomes. The cells include lymphocytes, monocytes, and macrophages.
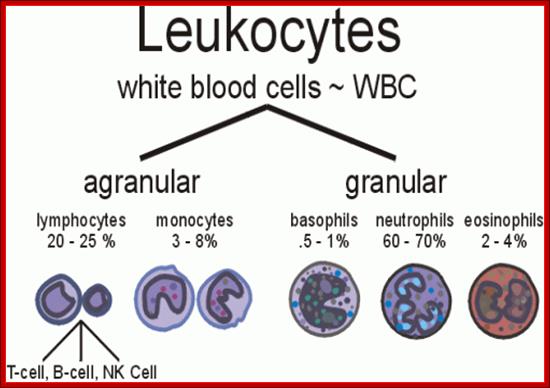
http://www.chxa.com

http://www.pinterest.com/; http://media-cache-ak0.pinimg.com/
Bones and Bone Marrow:
Bones are endowed with an hard outer structure that gives animals the hard skeletal network for them to stand erect and provide the basic structural features.� But the inner core contains liquid like substances called bone marrow in which large number of cells is found.� One of the major cell types is pluripotent stem cells; it is from this a variety of cells differentiate and move out into body circulation, ex. Red blood cells, white blood cells and many related cell types.
V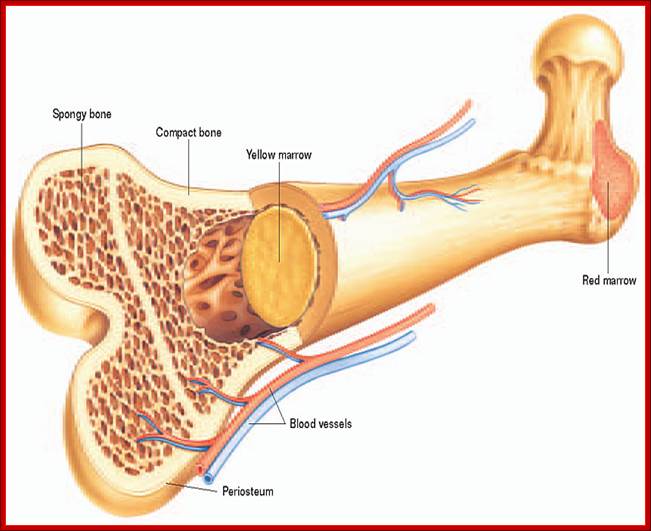
http://fbresearch.org/
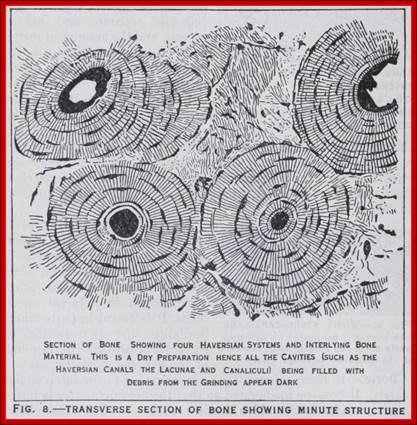
This fills the central cavity of tubular bones and the cavities of spongy bone tissue. It is largely composed of fat cells and is red or yellow in colour. Red marrow consists of fat cells lying in a tissue made up of large and small marrow cells and giant cells or myeloplaxes (fig. 9). The whole of these elements are supported in a delicate connective tissue. Some of the marrow cells are typical leucocytes and lymphocytes as found in circulating blood. Others (myelocytes) are larger than leucocytes, with round or oval nuclei, and a protoplasm containing small or large granules. These different types of cell probably develop into leucocytes. The giant cells are large spherical cells with several nuclei. In addition to fully developed red blood corpuscles there are other cell types.
Leukaemia; improving efficiency of bone marrow transplants:
�New information from research in Toronto could help improve the effectiveness of bone marrow transplants for patients suffering from leukaemia (and other cancers and immune disorders). Studies in mice, which were confirmed with samples from humans, showed that stem cells from bone ends are better at regenerating blood cells and immune system cells than the stem cells located in the shafts of bones. Not only are these cells better at regenerating, but they also work more efficiently and for longer periods of time than cells from the middle of the bones.
If doctors are able to collect stem cells that are more efficient, bone marrow transplants could not only be improved, but may be able to be effective for more people. The next step is to investigate the best ways of retrieving these superior stem cells. It�s exciting research, and could prove to really make a difference in bone marrow transplant methods. Stay tuned! http://fbresearch.org/
Bone Marrow � Structure and Function
by Dr Gauresh
Bone marrow is a highly cellular structure present within the hollow cavities of hard bone tissue. Bone marrow (Picture 1) is of 2 types, red bone marrow (produces blood cells) and yellow bone marrow (fatty tissue). The nature of bone marrow in different parts of the body changes with age. During childhood, bone marrow in all bones is red. In adulthood, the bone marrow cells in long bones of hand and leg become non-functional and are replaced by fat cells to form yellow bone marrow. The only bones to carry red bone marrow throughout life, are the vertebrae (back bones), sternum (breast bone), hip bone, and skull bones. Thus, any disease of bone marrow in adults is first seen in these bones!
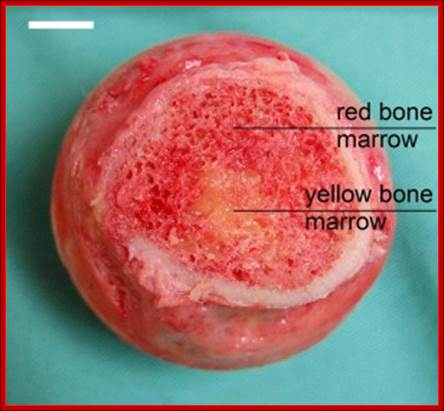
http://simple.wikipedia.org/
Picture: Types of bone marrow in cross section of a bone
(Source: Wikimedia Commons)
Bone marrow cells are highly functional and continuously divide and give rise to the different cells present in blood. This includes red blood cells, white blood cells, and platelets. The functions performed by each cell are
� Red Blood Cells (RBC)- transfer of oxygen from lungs to body tissues.
� White Blood Cells (WBC) � fighting infections by production of various types of cells (T Lymphocytes), chemicals, and antibodies (specific proteins against micro-organisms).
� Platelets � clotting of blood after any injury to prevent blood loss
The red marrow in bones consists of sponge like reticular frame work between long trabaculae. The space in this is filled with fat cells, stromal fibroblasts and blood precursor cells. These precursors mature and exit vascular sinuses into vascular circulation. Under emergency liver and spleen are recruited as sites for extra medullary haematopoiesis.
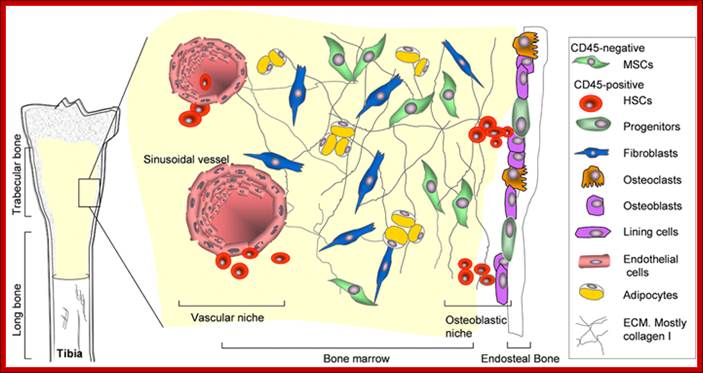
Cells that constitute the bone marrow stroma are:
� fibroblasts (reticular connective tissue)
� macrophages
� adipocytes
� osteoblasts
� osteoclasts
� endothelial cells forming the sinusoids
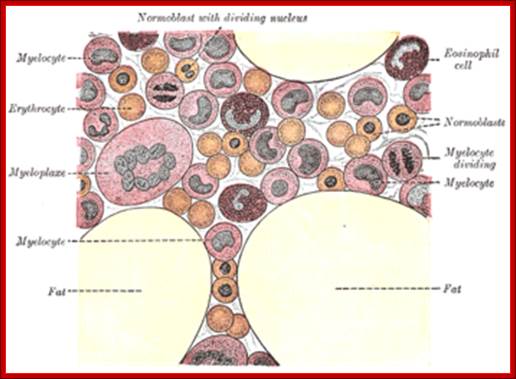
Bone Marrow from Grey anatomy; http://www.answers.com/
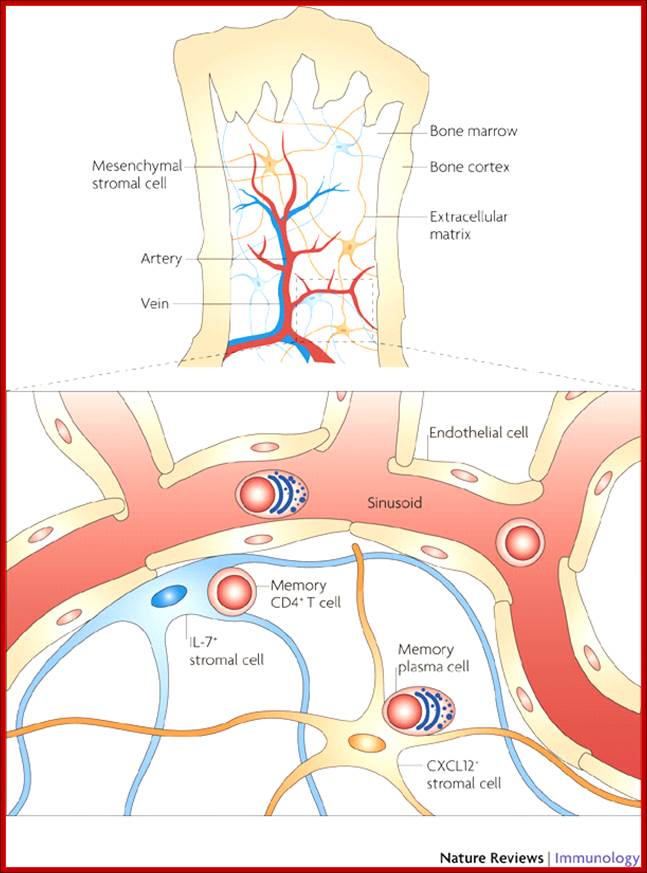
Bone marrow is the soft tissue that fills the cavities of the bone. The tissue is formed by mesenchymal stromal cells and the extracellular matrix they secrete. Haematopoietic progenitors and mesenchymal stem cells reside and move on this matrix. Memory CD4+ T cells and memory plasma cells also reside in the bone marrow; memory CD4+ T cells are maintained on interleukin-7 (IL-7)+ stromal cells and memory plasma cells are maintained on CXC-chemokine ligand 12 (CXCL12) stromal cells|
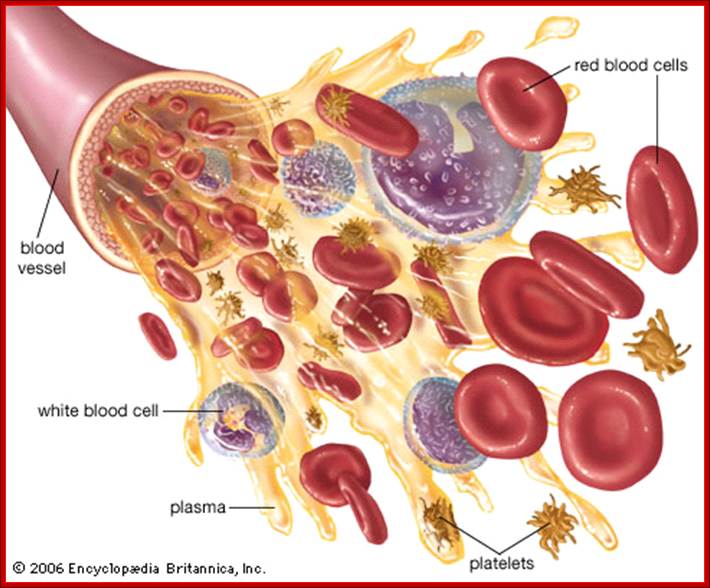
������������� Blood is made up of red blood cells, white blood cells, platelets, and plasma
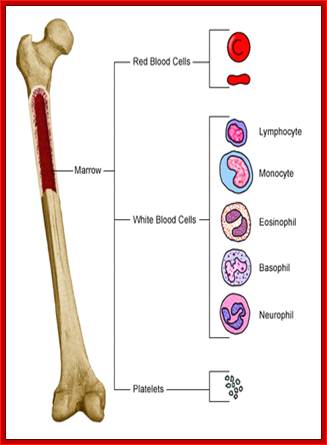
http://www.res.rcs.k12.tn.us/
Diagrams above and below are-Bone marrow derived cell types;
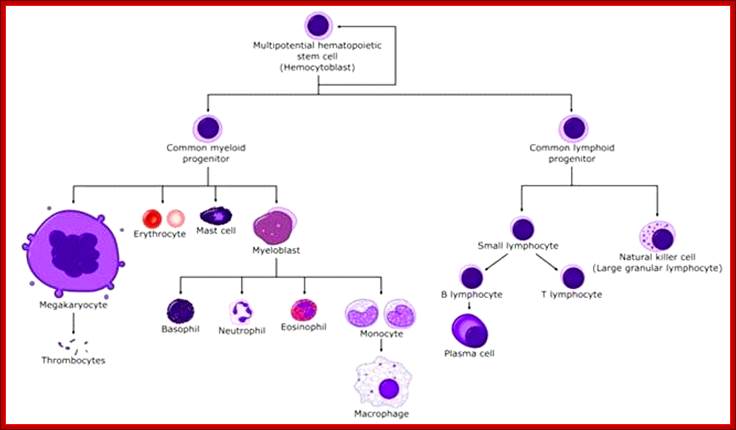
Multipotent stem cells in Bone Marrow differentiate into different cell types and emigrate.
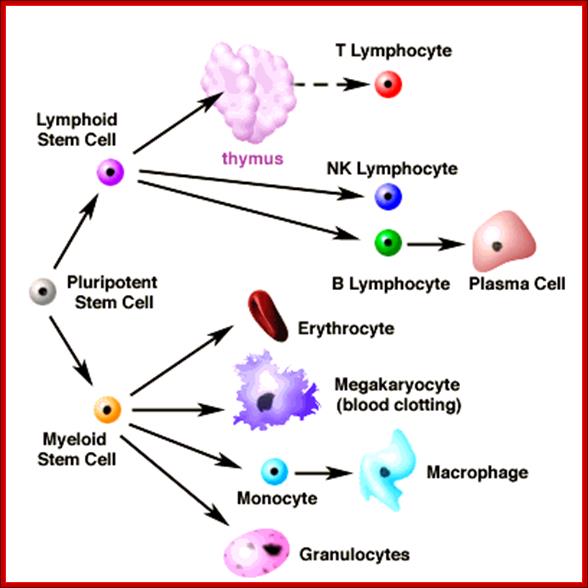
All blood cells originate from common stem cells and differentiate along particular lineages such as erythroid, megakaryocyte, granulocyte, myelocytic, and lymphocytic. Stem cells express two proteins, one CD34 and stem cell antigen -1, Sca1. These markers are used for separation of them from others cell types during bone marrow transplantation. Proliferation and maturation of these is triggered by cytokines. Many of the cytokines are colony stimulating factors. Hematopoietic cytokines are produced by stromal cells and macrophages in B/M (bone marrow). They are also produced when T-cells stimulated by antigens and cytokine or microbe activated, thus cells are replenished. The marrow also contains antibody producing plasma cells which are generated in peripheral lymphoid tissues in response to antigenic stimulation of B cells and then migrate to the marrow where they propagate and produce antibodies for many years.
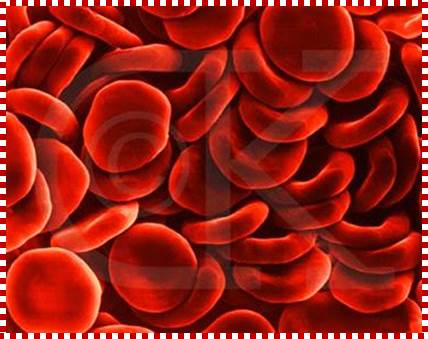
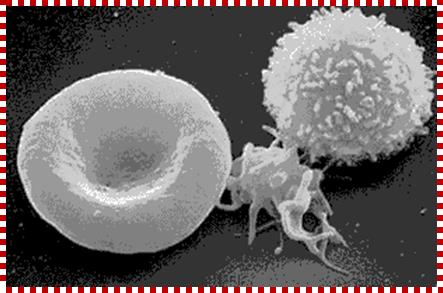
RBC and WBCs platelets; http://www.medindia.net/
Typical Lymphocyte-EM
The most important lymphocytes involved in immunity are T cells (thymus cells) and B cells (bursa-derived cells), they are the major cellular components of the adaptive immune response. T cells are involved in cell-mediated immunity whereas B cells are primarily responsible for humoral immunity (relating to antibodies) (WM).
|
Typical recognition markers for lymphocyte |
|||
|
CLASS |
FUNCTION |
PROPORTION |
PHENOTYPIC MARKER(S) |
|
Lysis of virally infected cells and tumour cells |
7% (2-13%) |
||
|
Release cytokines and growth factors that regulate other immune cells |
46% (28-59%) |
||
|
Lysis of virally infected cells, tumour cells and allografts |
19% (13-32%) |
||
|
Immunoregulation and cytotoxicity |
5% (2%-8%) |
||
|
Secretion of antibodies |
23% (18-47%) |
MHC class II, CD19 and CD21 |
|
In the circulatory system they move from lymph node to lymph node. This contrasts with macrophages, which are rather stationary in the nodes (wikimedia).
Thymus:
It is site for maturation of T cells. Thymus is bilobed structure in mediastinum; lobes are differentiated into outer cortex and inner medulla. Cortex contains a dense collection of T/L. Thymus also contains macrophages and dendrite cells (dendrites have markers CD8a).
Thyroid and Parathyroid in the neck
The Thymus: The thymus is where immature lymphocytes differentiate into T-lymphocytes. The thymus is fully formed and functional at birth. Characteristic features of thymic structure persist until about puberty, when lymphocyte processing and proliferation are dramatically reduced and eventually eliminated and the thymic tissue is largely replaced by adipose tissue. The lymphocytes released by the thymus are carried to lymph nodes, spleen, and other lymphatic tissue where they form colonies. These colonies form the basis of T-lymphocyte proliferation in the specific immune response. T-lymphocytes survive for long periods and recirculate through lymphatic tissues.
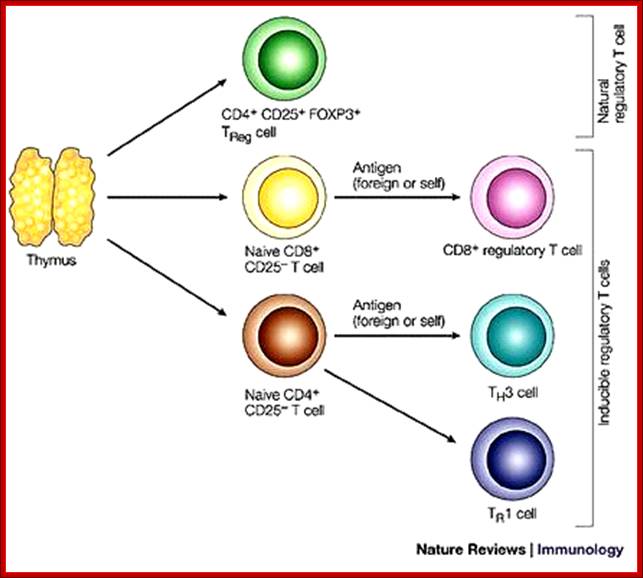
T- Lymphocytes mature into different cell types in Thymus
THYMUS HISTOLOGY. Thymus is an indispensable organ for the maturation of T-cells in fetal and early post-natal life. It is a lymphoid organ composed of two lobes connected to each other by an isthmus. Entire thymus is covered by a thin capsule. Two anatomically and functionally distinct regions can be identified, the outer cortex (top yellow arrow) and the inner medulla (yellow arrowhead). Fibrous septae arising from the capsule (bottom black arrows) penetrate as far deep as the corticomedullary junction creating numerous thymic lobules. Illustration of a single lobule in the thymus; its regions and cellular components. (Fig 7.15, Immunobiology, 7ed, � Garland Science 2008); http://gunningham.wordpress.com/
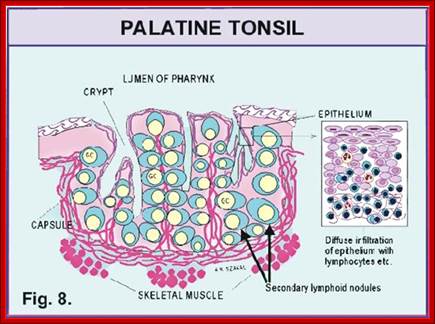
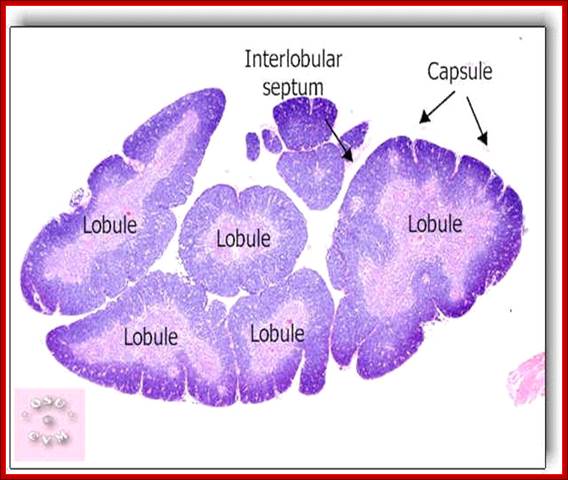
http://gunningham.wordpress.com/
The transformation of primitive or immature lymphocytes into T-lymphocytes and their proliferation in the lymph nodes is promoted by a thymic hormone called thymosin. Occasionally the thymus persists and may become cancerous after puberty and the continued secretion of thymosin and the production of abnormal T-cells may contribute to some autoimmune disorders. Conversely, lack of thymosin may also allow inadequate immunologic surveillance and thymosin has been used experimentally to stimulate T-lymphocyte proliferation to fight lymphoma and other cancers.
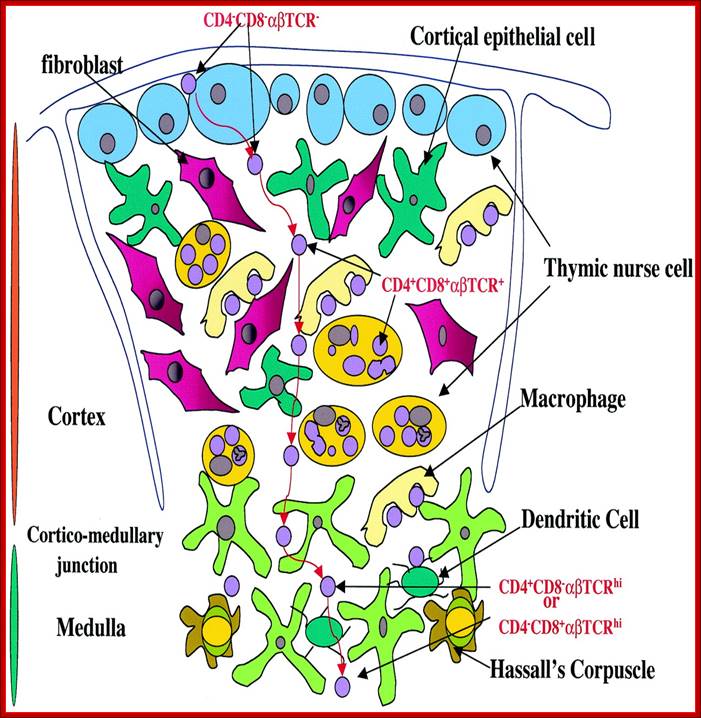
Diagram shows Thymus, the kind cell types found in Thymus; http://mmbr.asm.org/
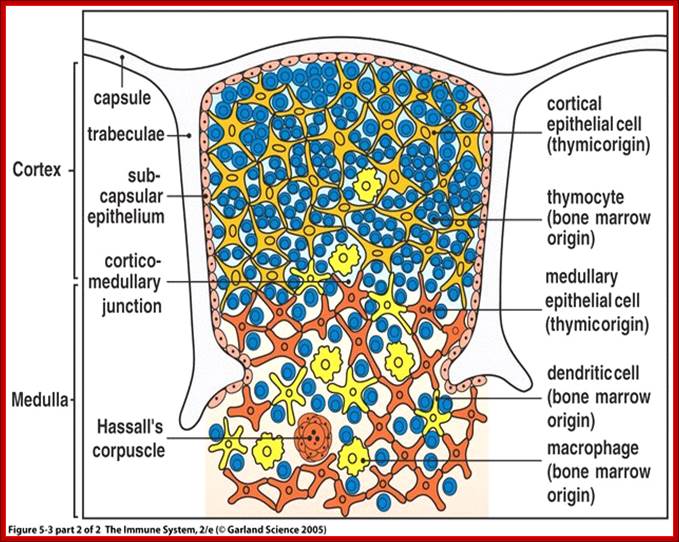
Various cell types found in Thymus glands; http://course1.winona.edu/
Spleen:
Spleen is major site for immune responses. The spleen filters the blood and reacts immunologically to blood-borne antigens. This is both a morphologic (physical) and physiologic process. In addition to large numbers of lymphocytes the spleen contains specialized vascular spaces, a meshwork of reticular cells and fibers, and a rich supply of macrophages which monitor the blood. Connective tissue forms a capsule and trabaculae which contain myofibroblasts, which are contractile. The human spleen holds relatively little blood compared to other mammals, but it has the capacity for contraction to release this blood into the circulation during anoxic stress. White pulp in the spleen contains lymphocytes and is equivalent to other large numbers of red blood cells that it lymph tissue, while red pulp contains filters and degrades.
The spleen functions in both immune and hematopoietic systems. Immune functions include: proliferation of lymphocytes, production of antibodies and removal of antigens from the blood. Hematopoietic functions include: formation of blood cells during fetal life, removal and destruction of aged, damaged and abnormal red cells and platelets, retrieval of iron from hemoglobin degradation, storage of red blood cells
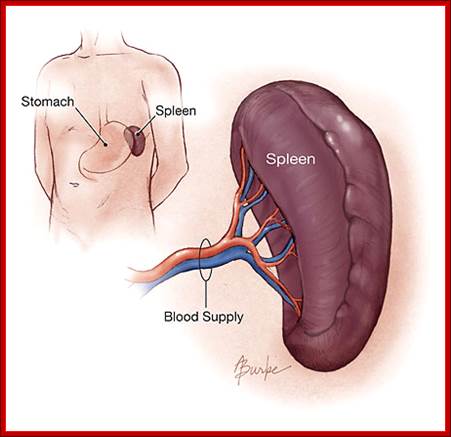
Position of the spleen in human body; http://www.whydoes.org/
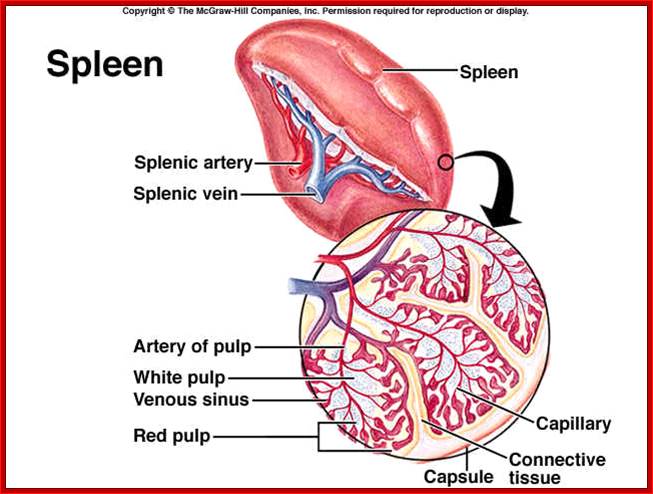
Spleen is the largest organ of lymphosystem.� It is the site for lymphocytes proliferation. Extracts aged and defective blood cells and platelets. Site for erythrocyte production of fetus, but capability may be restored in a crisis, stores platelets. http://www.lawrencegaltman.com/
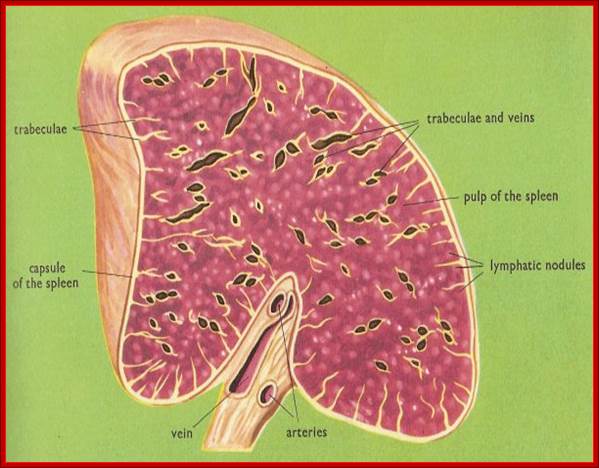
Spleen structural view
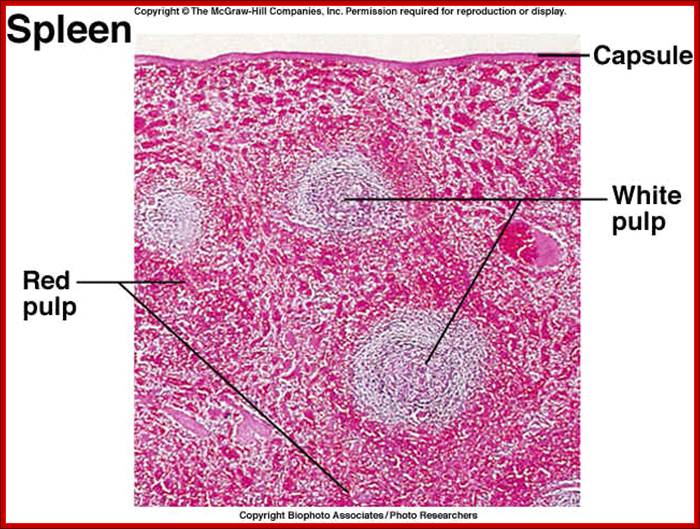 Spleen contains white pulp and red pulp with different functions
Spleen contains white pulp and red pulp with different functions
A lymphatic organ that lies behind the stomach, high up on the left side of the abdomen, on a level with the ninth to eleventh ribs. The spleen of an adult human weighs about 170 grams (6 ounces), and is 13�15 cm (5�6 in.) long, 7.5 cm (3 in.) wide, and 4 cm (1.5 in.) thick. It is elongate-oval in shape and of a dull purplish color. Although the spleen is located near the stomach it plays no part in digestion but is concerned only with the blood and the lymph and their circulations. All vertebrates have a spleen.
The
spleen is similar to a lymph node in shape and structure but is much
larger. In fact, it is the largest lymphatic organ in the body. It is enclosed
in an elastic capsule of connective tissue, which extends inward to divide the
organ into lobules.
The spleen consists of two types of tissue called white pulp and red
pulp. The white pulp is lymphatic tissue consisting mainly of lymphocytes around arteries. The red pulp consists of venous
sinuses (cavities) filled with blood and cords of lymphatic cells, such as
lymphocytes and macrophages.
Blood enters the spleen through the splenic artery, moves through the sinuses where it is
filtered, then leaves through the splenic vein.
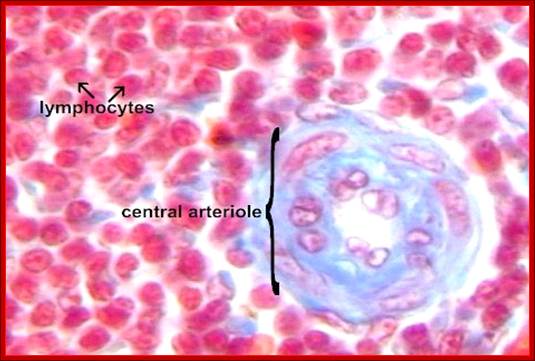
The many lymphocytes in the white pulp function as part of the specific immune defence.
Cutaneous immune system:
The skin is a specialized tissue and contains its own lymphocyte system and APCs. Schmitt D. Source; INSERM Unit� 346, Clinique Dermatologique, H�pital Edouard-Herriot, Lyon, France.
Abstract
The skin is not only a physico-mechanical barrier between the environment and the body, but it also functions as an immune organ. The immunological function of epidermis is principally linked to the presence in this tissue of a distinct subpopulation of dendritic cells: the Langerhans cells (LC). LC constitutes 2-4% of epidermal cell population and within epidermis they are the only cells which express MHC class II antigens constitutively. LC play a key role in the initiation of T cell responses to cutaneous antigens by picking up the antigen and migrating to the draining lymph node where they trigger specific T cell activation. There is also evidence that keratinocytes participate in immune responses in the skin since these cells produce a wide variety of cytokines that can modulate T cell responses. Dendritic cells comprise a system of highly efficient antigen-presenting cells which initiate immune responses such as the sensitization of T cells restricted by major histocompatibility complex molecules, the rejection of organ transplants and the formation of T-cell-dependent antibodies. Dendritic cells are found in many non-lymphoid tissues, such as skin and mucosa (Langerhans cells), and they migrate after antigen capture through the afferent lymph or the bloodstream to lymphoid organs, where they efficiently present antigen to T cells. Dendritic cells are difficult to isolate and, although they originate from bone marrow their growth and differentiation are still poorly characterized. Granulocyte macrophage-colony stimulating factor (GM-CSF) favours the out-growth of dendritic cells from mouse peripheral blood. The cooperation between GM-CSF and tumour necrosis factor-alpha (TNF-alpha) is crucial for the generation of human dendritic/Langerhans cells from CD34+ haematopoietic progenitors. The availability of large numbers of these cells should now facilitate the understanding of their role in immunological regulation and disorder. Recent studies reported that after 2-3 days in vitro incubation, both murine and human LC undergo profound phenotypic changes, as an enhancement in the expression of MHC class I and II antigens, LFA-3 and ICAM-1 molecules, a concomitant decrease of CD1a antigens and a loss of Fc gamma RII. Furthermore, cultured LC (cLC) lose or markedly reduce their specific cytoplasmic organelles: the Birbeck granules. Therefore, after a 2-3 days in vitro incubation, LC seem to acquire most of the features of lymphoid dendritic cells.
Mucosomal Immune system:
Gastro-intestinal tract and respirator surfaces contain lymphocytes and APCs. These respond to foreign pathogens that are ingested and inhaled. The epithelial layers of cells in these organs provide immunity. Perhaps the response is more or less immediate. Payer�s patches are found in epithelial layers. Mucosal system one of the largest surface area of the human body, starting from mouth to anus, nose to lungs and back, eyes-ears and urino-genital systems are layered with their special epithelial layers covering approximately 400m^2 surface.� This area is always subjected the attack of one or the other forms of antigenic pathogens, dust and environmental hazardous particles. This requires a robust immune system.� In fact the first line of defence starts from this system called mucosal immunity.� All these are associated with lymphoid tissues. It is estimated these layers not only act as mechanical barriers and have lymphoid system which act against pathogens.� These layers also respond to such infection with innate immunity system as the first line of defense.� They produce anti- microbial peptides secreted by the epithelial cells such as defensins, cathelicidins and histatins. epithelial defences are: 1) secretory IgA and IgM of limited antigen specificity, or natural antibodies, that trap the invading pathogen, 2) soluble pattern recognition receptors (PRRs) such as the mannose binding lectin (MBL), 3) the complement cascade components, 4) C-reactive protein, 5) lipopolysaccharide (LPS) binding protein (LBP) and CD14 (which can be also cell-associated), and 6) the cytokines and chemokines that orchestrate the immune system ( N. Aguilera Montilla et al).
Payer�s patches:
The Peyer�s patches (PP) are macroscopic lymphoid aggregates that are found in the submucosa along the length of the small intestine. Mature Peyer�s patches consist of collections of large B-cell follicles and intervening T-cell areas. B na�ve cells form the germinal centre of the follicle, supported or connected by follicular dendritic cells. These follicular dendritic cells are not bone marrow-derived and
are different from the dendritic cells that present antigens to the na�ve T cells. Each follicle is surrounded by a parafollicular area rich in T cells, where a large number of high endothelium venules exist, allowing cellular migration and lymphoid recirculation. Peyer�s patches differ from lymph nodes elsewhere in the body because they lack afferent lymphatics. This characteristic is in keeping with the notion that antigen is sampled from the lumen via the overlying epithelium ( N. Aguilera Montilla et al).
Payers Patches:
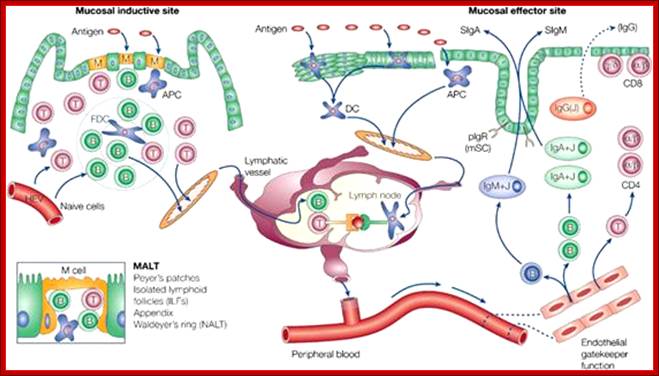
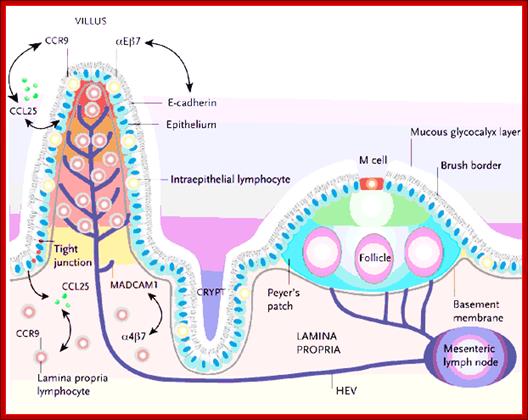
The organized tissues of the Peyer's patches and mesenteric lymph nodes (MLNs) are involved in the induction of immunity and tolerance, whereas the effector sites are scattered throughout the lamina propria and epithelium of the mucosa. Both the Peyer's patches and villus lamina propria are drained by afferent lymphatics that go to the MLNs. SED, subepithelial dome; TDA, thymus-dependent area.; http://www.nature.com/http://dc390.4shared.com/
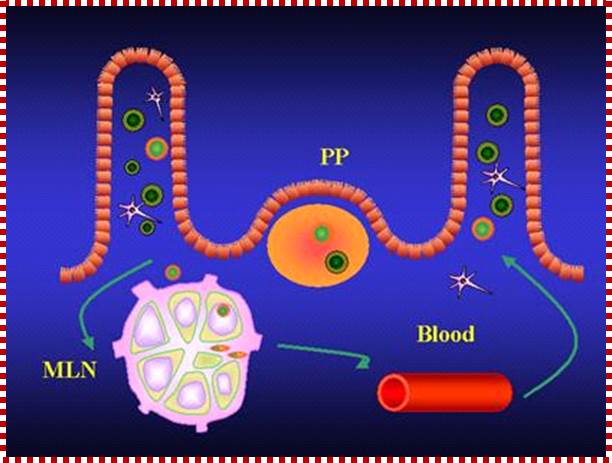
��������� Payers patches in mucosal system; http://chlamydiae.com/
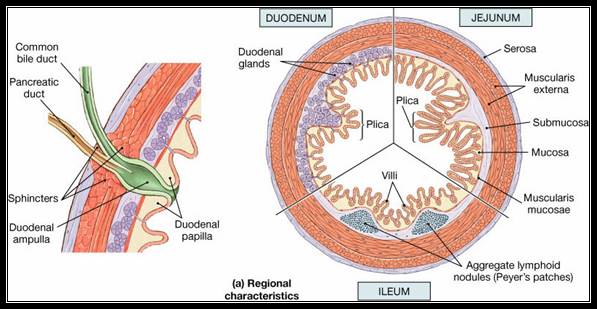
The last segment of the small intestines is about 3.5 m (12 ft) long and ends where it joins the cecum at the sphincter called the ileocecal valve. Both the jejunum and ileum are supported by a mesentery called the mesentery proper. Lymphoid nodules are more numerous in the lamina propria of the ileum and may form aggregated lymphoid nodules (Peyer�s patches).
Histology of the Small Intestines.
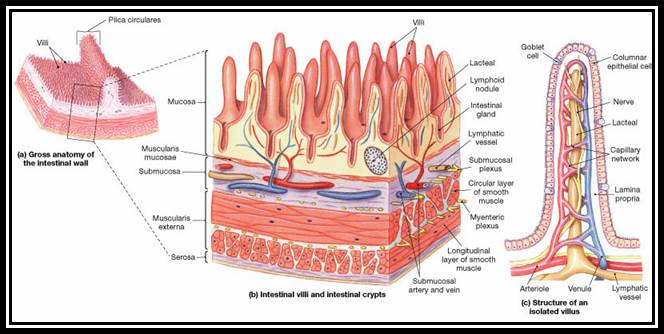
http://droualb.faculty.mjc.edu/
|
The mucosa of the small intestines has finger-like projections called villi (sing. villus). The epithelial cells of the villi have microvilli on at their apical surface. Goblet cells that secrete mucins are found among the epithelial cells. The plicae circulares, villi and microvilli increase the surface area enormously. |
|
Tubular intestinal glands are found between the villi extending into the lamina propria. Cells in the intestinal glands continually divide and migrate onto the villi where they are eventually shed at the tips of the villi. |
|
The lamina propria of the small intestines have lymphatic vessels called lacteals that transport absorbed lipids to the circulation. |
���������������������������������������� Payer�s patches
Basic structure
The gastrointestinal tract is a muscular tube lined by a special layer of cells, called epithelium. The contents of the tube are considered external to the body and are in continuity with the outside world at the mouth and the anus. Although each section of the tract has specialized functions, the entire tract has a similar basic structure with regional variations.
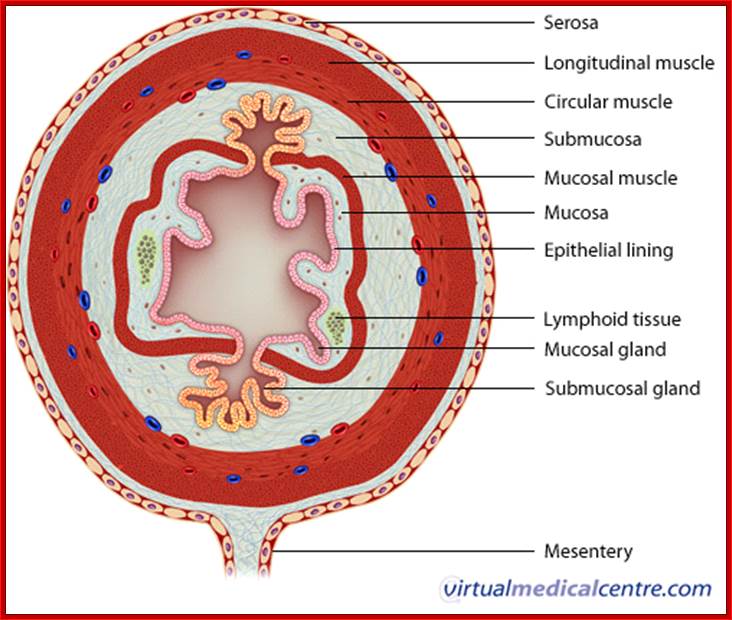
The wall is divided into four layers as follows:
Mucosa:
(MYOO-kus MEM-brayn) The moist, inner lining of some organs and body cavities (such as the nose, mouth, lungs, and stomach). Glands in the mucous membrane make mucus (a thick, slippery fluid). Also called mucosa.
The innermost layer of the digestive tract has specialized epithelial cells supported by an underlying connective tissue layer called the lamina propria. The lamina propria contains blood vessels, nerves, lymphoid tissue and glands that support the mucosa. Depending on its function, the epithelium may be simple (a single layer) or stratified (multiple layers).
Areas such as the mouth and oesophagus are covered by a stratified squamous (flat) epithelium so they can survive the wear and tear of passing food. Simple columnar (tall) or glandular epithelium lines the stomach and intestines to aid secretion and absorption. The inner lining is constantly shed and replaced, making it one of the most rapidly dividing areas of the body! Beneath the lamina propria is the muscularis mucosa. This comprises layers of smooth muscle which can contract to change the shape of the lumen.
Submucosa
The Submucosa surrounds the muscularis mucosa and consists of fat, fibrous connective tissue and larger vessels and nerves. At its outer margin there is a specialized nerve plexus called the Submucosa plexus or Meissner plexus. This supplies the mucosa and submucosa.
Muscularis externa;
This smooth muscle layer has inner circular and outer longitudinal layers of muscle fibres separated by the myenteric plexus or Auerbach plexus. Neural innervations control the contraction of these muscles and hence the mechanical breakdown and peristalsis of the food within the lumen.
Serosa/mesentery
The outer layer of the GIT is formed by fat and another layer of epithelial cells called mesothelium.
Lymphoid system:
Lymph is a clear, watery fluid that transports waste products and proteins out of the spaces between the cells of the body tissues. It also destroys bacteria or other pathogens that are present in the tissues. Lymph originates from blood containing arteries at the capillary regions, it moves out as it slows n capillary regions. This slowing allows some plasma to leave the arterioles and flow into the tissues where it becomes tissue fluid.
Lymphatic capillaries;
|
|
|
Blood capillaries allow fluid to leave, @ Lymph Notes.Com |
In order to leave the tissues, the lymph must enter the lymphatic system through specialized lymphatic capillaries. Approximately 70% of these are superficial capillaries located near, or just under, the skin. The remaining 30%, which are known as deep lymphatic capillaries, surround most of the body�s organs.
Lymphatic capillaries begin as blind-ended tubes that are only a single cell in thickness. These cells are arranged in a slightly overlapping pattern, much like the shingles on a roof. Each of these individual cells is fastened to nearby tissues by an anchoring filament.
As shown in the animation below, pressure from the fluid surrounding the capillary forces these cells to separate for a moment to allow lymph to enter the capillary. Then the cells of the wall close together. This does not allow the lymph to leave the capillary. Instead it is forced to move forward.
� Also known as extracellular fluid, this is fluid that flows between the cells but is not found within the cells. This fluid delivers nutrients, oxygen, and hormones to the cells.
� As this fluid leaves the cells, it takes with it cellular waste products and protein cells.
� Approximately 90% of this tissue fluid flows into the small veins. Here it enters the venous circulation as plasma and continues in the circulatory system.
� The remaining 10% of the fluid that is left behind is known as lymph.
� Lymphoid system consists of lymph vessels and lymph nodes. It acts third parallel fluid transport system in the body Lymph vessels starts at interstitial spaces all over the body, start as capillaries with closed ends. Such capillaries with one another join into small vessels. Such vessels or ducts join with one another. While they run they pass through lymph nodes. Such collected larger ducts, one right lymph duct empty its contents into right subclavian vessels. The thoracic duct empties into left subclavian vein. Lymphatic nodes play a very important role in activating lymphocytes and other immune cells, which remove infected pathogens or toxic materials. The lymphatic system aids the immune system by filtering waste out of the lymph. This allows the lymph to safely return to the circulatory system.
� The lymphatic system removes pathogens, excess fluids, waste products, and debris, dead nutrients, oxygen, and hormones from the plasma (arterial blood without cells within the tissues.
� When lymphedema develops it causes swelling it causes the affected tissues and the fluid is stagnant (unable to flow) and it does not drain properly. (Pathogens are microorganisms that cause diseases.)
� When bacteria enter the lymph flow through a break in the skin, they thrive on this protein-rich fluid. It is for this reason that lymphedema affected tissues are prone to infections.
� Blood cells, cancer cells, and toxins those are present in the interstitial fluid between the cells within the tissues. (Pathogens are microorganisms that cause diseases.)
� The lymphatic system works in close cooperation with the circulatory system to deliver
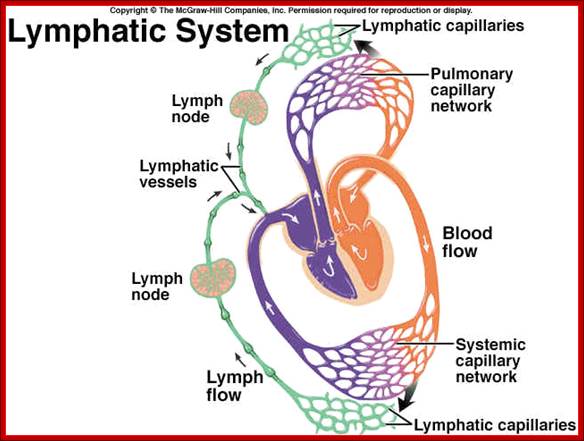
� Cervical lymph nodes - Located in the head and neck portion. In total, they are six in number.
� Axillary lymph nodes - Present in the underarm area. They are divided into two types, namely, superficial and deep lymph nodes.
� Supraclavicular lymph nodes - Situated along the collarbone or clavicle.
� Femoral lymph nodes - Located in the upper thigh portion, along the femoral veins.
� Mesenteric lymph nodes - Distributed in the lower abdomen.
� Mediastinal lymph nodes - Present between the air sacs of the lungs.
� Inguinal lymph nodes - Located in the groin area. They may be supercritical or deep lymph nodes.
Lymphocyte circulation and homing of the same:
Vascular system provides the route for the circulation of lymphocytes all the way from heart to the extreme peripheral regions of the body, a great supply system and also a collecting system for recirculating the blood. Thus the circulation of lymphocytes from one peripheral system to the other is greatly facilitated. This also facilitates the movement to inflammatory sites. Some of them move to only specific sites but not to others, so it is called homing. This homing is aided by adhesion of molecules on lymphocytes, endothelial cells and ECM and chemokines produced by endothelial cells.
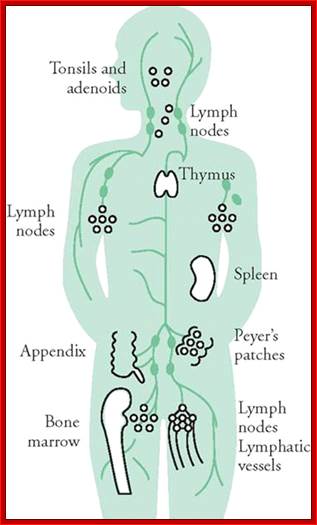
�������������������������������������������������� Position of immune related structures
Na�ve L/C preferentially home and recirculate via peripheral lymphoid organs, where they reorganize and respond to antigens they encounter. Memory T-cells and effecter cells move out of lymph nodes and home in peripheral tissues where the infection has occurred. They encounter the pathogenic antigens and they have to act to eliminate such infection; this phase is called effector phase of adaptive immune response.
Memory T-cells are heterogeneous in terms of their expression of adhesion molecules and chemokines receptors. They have the facility or propensity to migrate to different tissues.
Organization and distribution of immune cells or immune system as a whole in the body for eliciting immune response is critical and important. Quick response to infection and migration of immune cells to the site of infection is crucial.
The adaptive immune response takes time, when compared to innate immune response; for this immune response, though slow, it is very strong and permanent, for it is mediated by specific cell types.
Immune cells such as B and T lymphocytes generate specific antigen recognizing receptors and they are responsible for action against the antigens and generate memory cells of adaptive immune system. NK cells are distinct types of lymphocyte system and they do not express highly diverse antigen receptors. Lymphocytes with their diverge cellular and functional characters express different subsets of surface molecules and such surface proteins are expressed even on leukocytes, and they are named accordingly as clusters of differentiation (CDs).
Both T and B/cells take their origin in B/M. B/cell development progresses in B/M and T/cells migrate to Thymus and mature. After maturation both leave their site of maturation and enter into circulation and populate peripheral lymphoid organs.
Na�ve cells are those that are not stimulated by any antigens. Only on stimulation by antigens they differentiate into effector cells. Effector B cells secrete antibodies and T cells include cytokine secreting cells, CD4 helper cells and CD8 CTL cells.
Some of the progeny of antigen-activated B and T/ls differentiate into memory cells and they remain for a long period of time and respond very quickly on second exposure to antigens.
Antigen presenting cells (APCs), include dendrite cells, mononuclear phagocytes and follicle dendrite cells. They transport antigens onto the surface of cells so lymphocytes can identify and lock on to the same and get activated.
The organs of immune system are bone marrow and thymus; they act as generative tissues, and lymph nodes and spleen act as peripheral organs, where the na�ve L/Cs get activated by antigens.
Stem cells are found in bone marrow which in time proliferate and differentiate into blood cells and lymphocytes. With the exception of T cells all others mature. T cells mature in thymus.
T and B cells responds to antigens in lymph nodes for the antigens are collected during lymph draining peripheral tissues. Lymphocytes respond to blood borne antigens in spleen. Lymph nodes and spleen are organized into follicular B zones and T zones. T-zones act as home for the mature dendrite cells. In fact dendrite cells are specialized for activation of na�ve T cells. FDC reside in B zone and serve to activate B cells during humoral immune response to protein antigens. Cytokines induce the development of secondary lymphoid tissue architecture.
Lymphocytes and APC cells which are adapted to respond to environmental antigens encountered in skin are found in the same region. A network of Langerhans, which are immature dendrite cells are found in the epidermal layers of skin which serve to trap antigens and transport them to draining lymph nodes. The mucosal system includes specialized collection of lymphocytes and APC which are organized to optimize encounters with environment that are introduced via respiratory and gastrointestinal tracts.
Lymphocytes move from one site to the other all the time through blood and lymphatic vessels; it is very important for effector activity of immune response.
Immature T cells, called Naive cells recirculate among peripheral lymphoid organs. This increases the probability to encounter antigens displayed by APC cells such as mature dendrite cells. This happens when na�ve T cells are in recirculation. Effector T cells are recruited to the site of inflammation by microbe infection. Memory cells enter lymphoid organs or peripheral tissues.
Chemokines called chemo-attractant proteins facilitate lymphocyte recirculation; this also helps in adhesion of molecules on lymphocytes. This is called homing of receptors and their ligands on vascular endothelial cells called addressins. Endothelial cells in different tissues may express different ligands for homing receptors that promote tissue-specific lymphocytes homing.
Different population of L/C exhibit distinct pattern of homing. Na�ve T cells migrate probably to lymph nodes which are mediated by their binding of L-selectin on T cells to be destined to peripheral lymph node addressins found in endothelial venules in lymph nodes and by CCR7 receptors on T cells that bind to chemokines produced by lymph nodes. The effector and memory T cells that are generated by antigen stimulated T cells that exit lymph nodes. They have decreased L-selectins and CCR7 expression but increased expression of integrins and E-selectins and P-selectin ligands. These mediate binding of endothelium at peripheral inflammatory sites. Effector and memory lymphocytes also express receptors for chemokines that are produced in peripheral tissues.
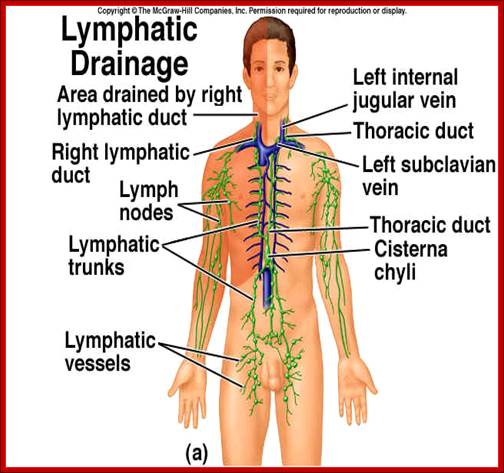
Lymph system is almost parallel to blood circulatory system; http://faculty.southwest.tn.edu/
Circulatory system-http://en.wikipedia.org/
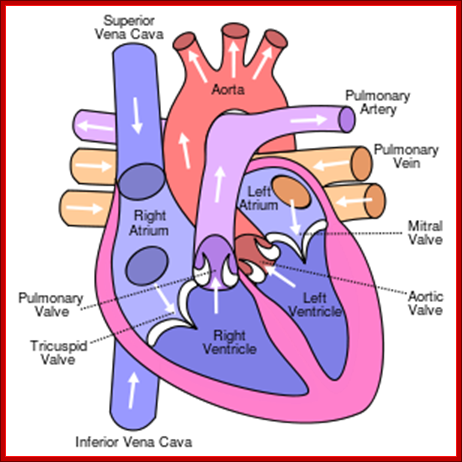
View from the front, which means the right side of the heart is on the left of the diagram (and vice-versa); http://en.wikipedia.org/
http://www.wellsphere.com/ http://intranet.tdmu.edu.ua/
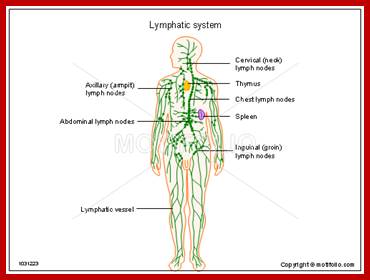
http://www.motifolio.com
The lymph system works parallel to blood vascular system. The vascular system collects blood from various parts of the body down loads into the heart, similarly the blood also flows from the heart to other parts of the body, an ingenious network; take a look at the blood vessel network in human head
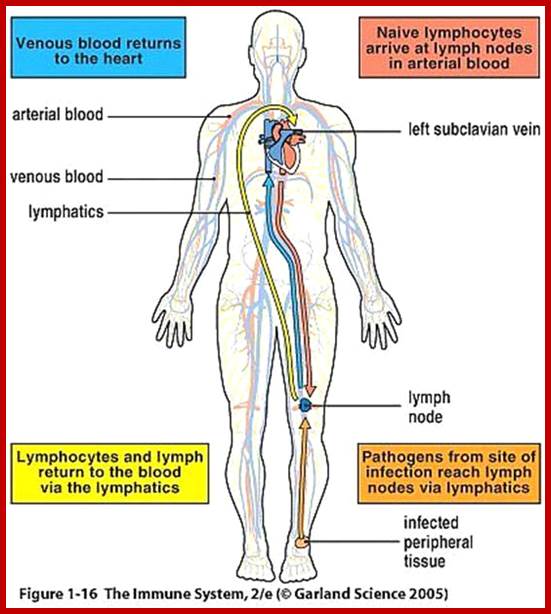
Garland Science 2005
���������������������������������������� Artistic depiction of the lymphatic system including lymph nodes and lymph vessels. Red illustrates the concept that particular lymph nodes may be involved, depending on the disease. Nodes and Nodules: https://www.verywell.com; http://www.canstockphoto.com
Functions of the lymphatic system:
1) To maintain the pressure and volume of the extracellular fluid by returning excess water and dissolved substances from the interstitial fluid to the circulation.
2) Lymph nodes and other lymphoid tissues are the site of clonal production of immuno-competent lymphocytes and macrophages in the specific immune response.
Filtration forces water and dissolved substances from the capillaries into the interstitial fluid. Not all of this water is returned to the blood by osmosis, and excess fluid is picked up by lymph capillaries to become lymph. From lymph capillaries fluid flows into lymph veins (lymphatic vessels) which virtually parallel the circulatory veins and are structurally very similar to them, including the presence of semi lunar valves.
Lymphokinetic motion (flow of the lymph) due to:
1) Lymph flows down the pressure gradient.
2) Muscular and respiratory pumps push lymph forward due to function of the semilunar valves
The lymphatic veins flow into one of two lymph ducts. The right lymph duct drains the right arm, shoulder area, and the right side of the head and neck. The left lymph duct, or thoracic duct, drains everything else, including the legs, GI tract and other abdominal organs, thoracic organs, and the left side of the head and neck and left arm and shoulder.
These ducts then drain into the subclavian veins on each side where they join the internal jugular veins to form the brachiocephalic veins.
Lymph nodes lie along the lymph veins successively filtering lymph. Afferent lymph veins enter each node, efferent veins lead to the next node becoming afferent veins upon reaching it.
Lymph nodes:� They are small nodules with aggregates of lymphocyte rich tissue found along lymphatic channels. Lymph nodules differentiated into cortex and medulla. Lymph nodes are encapsulated by thick capsule pierced by efferent (going out) and afferent (entering into) lymphatic vessels. They are connected by arteries (incoming) and a single vein (outgoing). Follicles are found as islands where B/L is found; stimulated B-cells proliferate and generate memory cells. T/l is located in beneath the cortex in medulla. Most of the T/cells are CD4 helper cells intermingled with CD8 cells. Dendrite cells are also centralized in T/cell zone. Lymph nodes: Lymph nodes are small encapsulated organs located along the pathway of lymphatic vessels. They vary from about 1 mm to 1 to 2 cm in diameter and are widely distributed throughout the body, with large concentrations occurring in the areas of convergence of lymph vessels. They serve as filters through which lymph percolates on its way to the blood. Antigen-activated lymphocytes differentiate and proliferate by cloning in the lymph nodes.
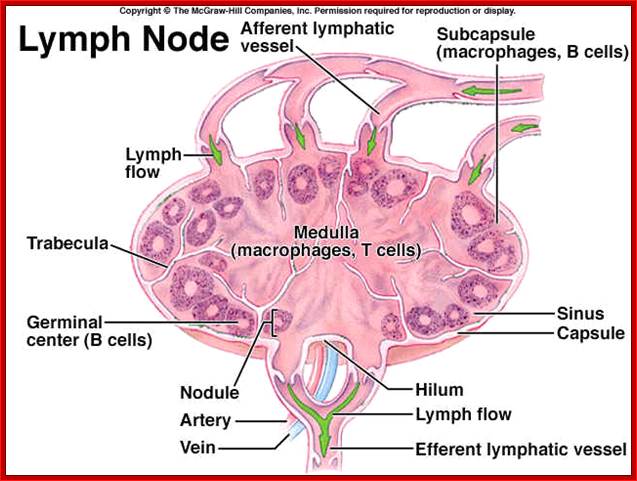
Lymph nodes play a pivotal role immune system; http://www.mhhe.com/
There
are at least 600-650 lymph nodes are present in human body. Lymph nodes
act like filters for the lymph
fluid. The fluid travels through the
reticular tissue and endures the filtering process via the hundreds of lymph
nodes which line the lymphatic vessels. Phagocyte cells line the reticular
tissue as well, which assist in the filtering process of the lymph fluid. The main function of lymph nodes is to act as a drainage system
by absorbing and expelling proteins, dead cells, bacteria and other waste
products from the body. Basically they clean and filter the lymph before it is
returned to the blood.
They are also a major part of the immune system, as they produce antibodies and
lymphocytes.
If there is an infection present in the body, the lymph nodes produce more
lymphocytes to combat the infection.
http://wiki.answers.com/Q/What_are_the_functions_of_the_lymph_nodes#ixzz1xGu9lQdG
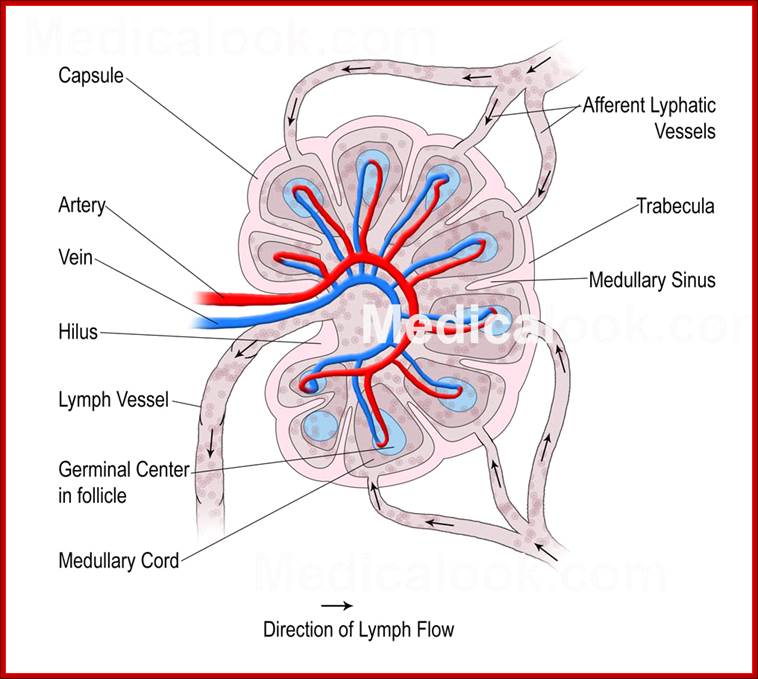 �http://www.medicalook.com
�http://www.medicalook.com
The anatomic segregation of different classes of lymphocytes in distinct areas of the node is dependent on cytokines. And antigens are transported to lymph nodes mainly through lymphatic vessels.
Diffuse Lymphatic Tissue and Lymphatic nodules: The alimentary canal, respiratory passages, and genitourinary tract are guarded by accumulations of lymphatic tissue that are not enclosed by a capsule (i.e. they are diffuse) and are found in connective tissue beneath the epithelial mucosa. These cells intercept foreign antigens and then travel to lymph nodes to undergo differentiation and proliferation. Local concentrations of lymphocytes in these systems and other areas are called lymphatic nodules. In general these are single and random but are more concentrated in the GI tract in the ileum, appendix, cecum, and tonsils. These are collectively called the Gut Associated Lymphatic Tissue (GALT). MALT (Mucosa Associated Lymphatic Tissue) includes these plus the diffuse lymph tissue in the respiratory tract.
Lymphatic Vessels:
Parts of the blood plasma will exude from the blood vessels into the surrounding tissues because of transport across the endothelium or because of blood pressure and the fenestration of some capillaries (this process is partly counteracted by the higher osmotic pressure of the blood). The fluid entering tissues from capillaries adds to the interstitial fluid normally found in the tissue. The surplus of liquid needs to be returned to the circulation. Lymph vessels are dedicated to this unidirectional flow of liquid, the lymph. Three types of lymph vessels can be distinguished based on their size and morphology.
Lymph capillaries:
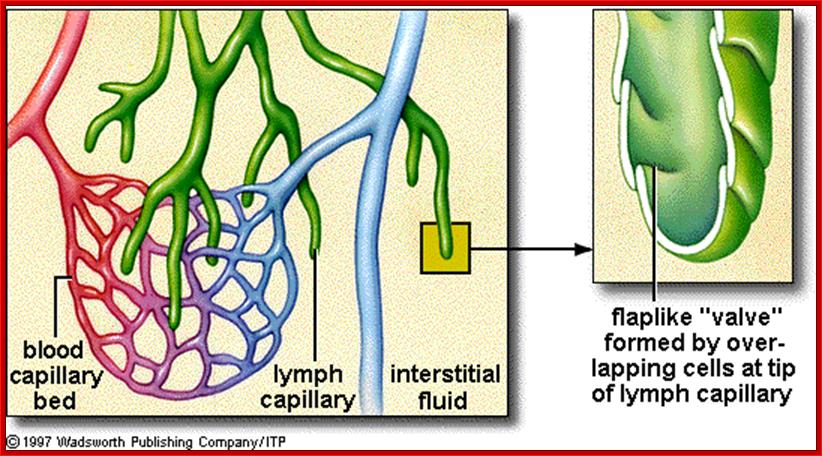
The lymph vascular system starts at capillary beds. Interstitial fluid enters the lymph capillaries. These vessels have no pronounced entrance. Instead, water and solutes move in at their tips; http://www.student.loretto.org/
They are somewhat larger than blood capillaries and very irregularly shaped. They begin as blind-ending tubes in connective tissue. The basal lamina is almost completely absent and the endothelial cells do not form tight junctions, which facilitates the entry of liquids into the lymph capillary. Temporary openings in the endothelial lining of the lymph capillaries also allow the entry of larger particles into the lymph capillaries (lipid droplets, which are absorbed from the lumen of the gut do not enter blood capillaries, but enter the circulation via lymph vessels which are found in the villi of the ileum and jejunum). Lymph capillaries merge to form
Lymph collecting vessels:
They are larger and form valves but otherwise appear similar to lymph capillaries. The lymph is moved by the compression of the lymph vessels by surrounding tissues. The direction of lymph flow is determined by the valves. Lymph vessels empty intermittently into lymph nodes from which the lymph continues in efferent lymph vessels.
Only very little lymph is returned from the limbs if they are immobilized, which illustrates the importance of muscular action in lymph transport. This is also the reason for immobilizing limbs that are either infected or that have been bitten by venomous Australians. The effect can also be observed after long intercontinental flight when you may feel that your shoes and socks are just about one number too small. Finally, impeded lymph drainage is one of the problems associated with surgery which requires the removal of lymph nodes and which thereby interrupts the lymph collecting vessels.
Eventually the lymph collecting vessels merge to form Lymph ducts which contain one or two layers of smooth muscle cells in their wall (some textbooks call this layer the tunica media of lymph vessels). They also form valves. The walls of the lymph ducts are less flexible in the region of the attachment of the valves to the wall of the duct, which may give a beaded appearance to the lymph ducts. Peristaltic contractions of the smooth muscle contribute to the movement of lymph towards the heart in addition to the compression of the ducts by surrounding tissues.
The largest lymph duct of the body, the thoracic duct, drains lymph from the lower half and upper left quadrant of the body and empties the lymph into the circulation by merging with the vascular system close to the junction of the left internal jugular and subclavian veins. That it is the largest lymph duct does not mean that it is a large vessel when compared to the large arteries and veins. It actually is not much larger (about 5mm in diameter) than one of the superficial forearm veins.
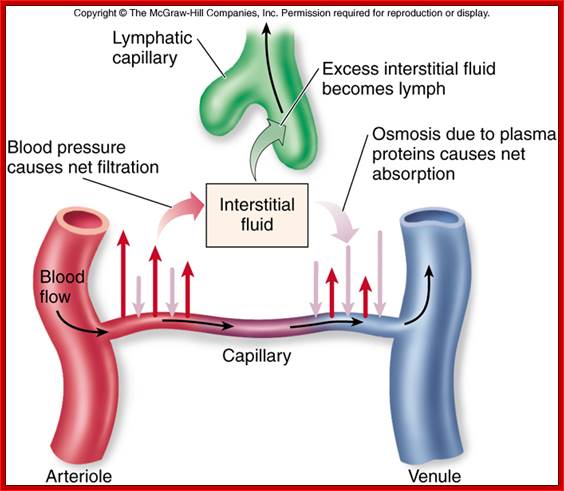
Circulatory system: http://www.bio.utexas.edu/
Start at blood vessel capillaries as blunt tubes and join with one another. In this interstitial cells, fluid and cells start moving into capillaries. Capillaries are lined with a layer of endothelial cells with gaps in between them. The vessels are supported by very thin muscle system. The fluid enters though osmotic pressure.
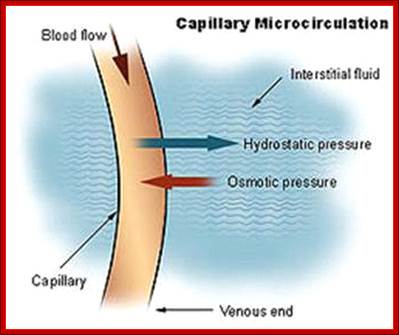
www.rocketswag.com
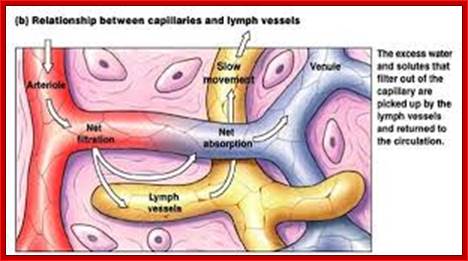
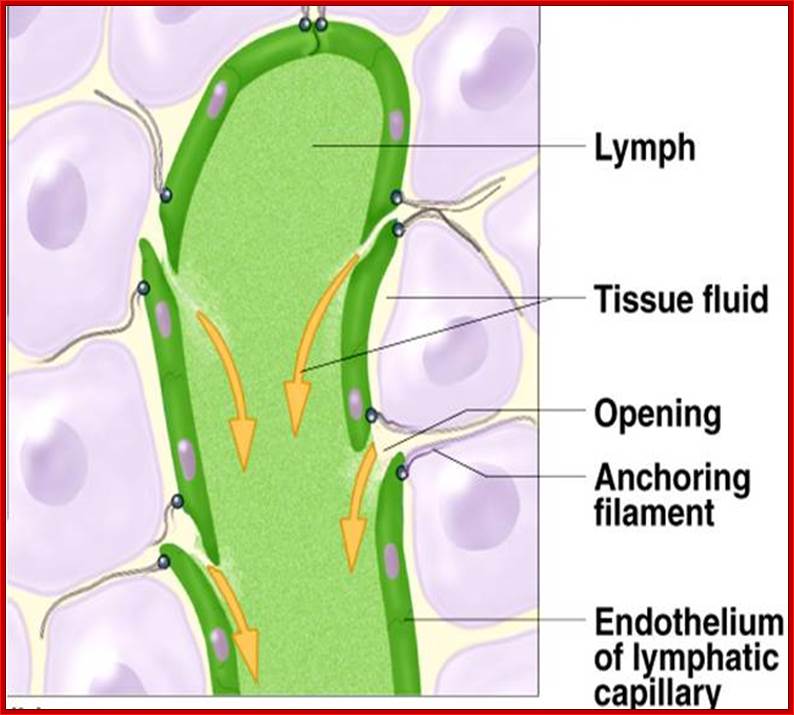
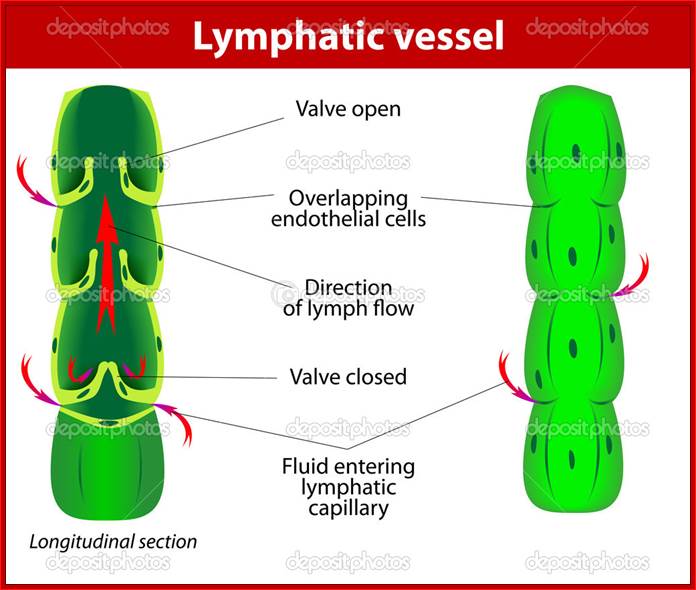
http://www.biologyaspoetry.com/
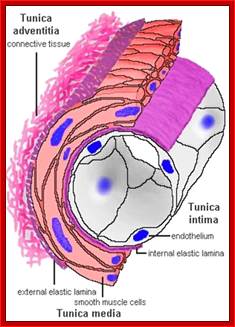
https://legacy.owensboro.kctcs.edu
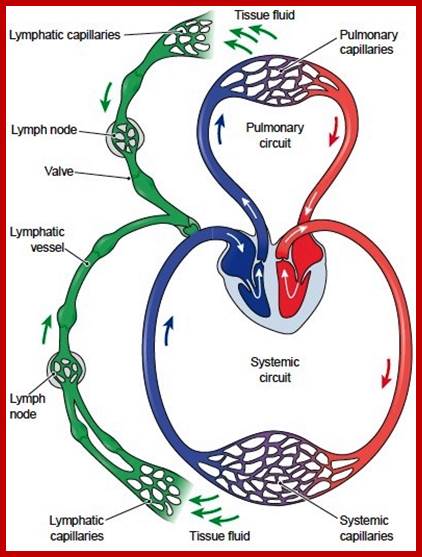
http://encyclopedia.lubopitko-bg.com
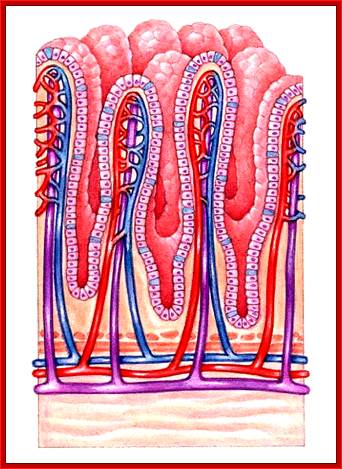 �
�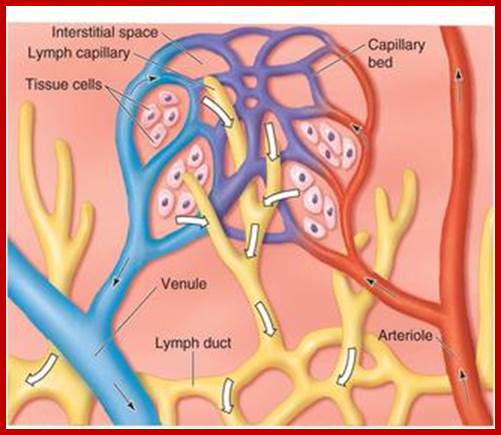
Lymph capillaries collecting lymph; https://humanphysiology2011
Lymph Nodes:
Lymph nodes look like small ball like structure of 1-2cm size located though out our body.� They are linked by lymph vessels.� Lymph nodes act as garrisons of B and T and other immune cells.� They are loaded with lymphocytes and macrophages (WBCs).� In response to antigens lymphocytes in nodules respond and secrete antibodies that are circulated.
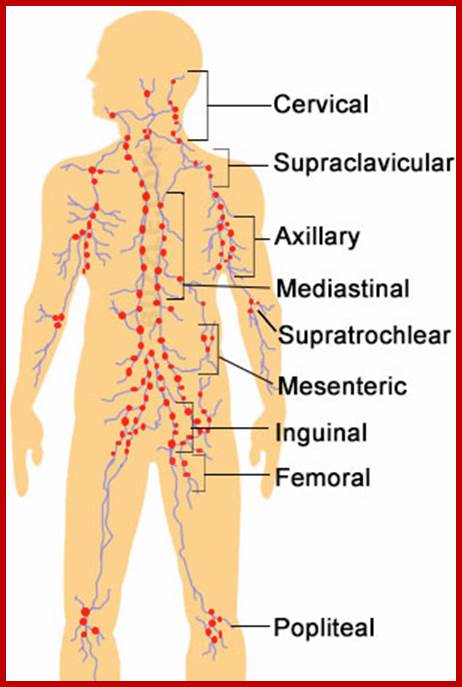
http://www.buzzle.com
Lymph nodes are essential organs of the immune system and play a
crucial role in the normal functioning of the system. Here are the major
functions of lymph nodes:
Read more at BuzzleLymph node; showing�
capsule, subcapsular sinus, �germinal centers, lymphoid nodule, �trabeculae; Popliteal lymph nodes; Femoral lymph nodes; Inguinal lymph
nodes:; Mesenteric lymph nodes: Supratrochlear lymph nodes; Axillary lymph
nodes; Supraclavicular lymph nodes: Cervical lymph nodes:
http://www.buzzle.com/articles/lymph-nodes-locations-and-functions.html
Lymph nodes are encased in a fibrous capsue that extend as trabaculae. Encased node is divided in to outer cortex and inner core Meddulla.� It is also studded with reticular fibers with in which one finds WBC including dentrite cells and macrophages and lymphocytes. Nodules have system for the� inflow (afferent system) and outflow (efferent system) of lymph vessels.
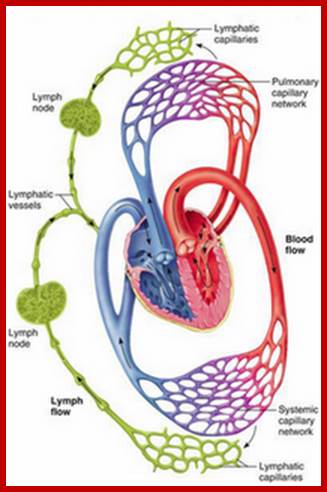
The lymphatic system includes lymph, lymphocytes, lymph vessels, lymph nodules, lymph nodes, tonsils, spleen and thymus gland. Part of the body�s defense system, the lymph nodes filter lymph and the spleen filters blood, removing microorganisms and other foreign substances. The lymph nodes act as a filtration system that keeps particulate matter such as bacteria from entering the bloodstream. They produce both lymphocytes and monocytes. Lymph tissue contains lymphocytes and other cells that can destroy microorganisms and foreign substances. The cells have obtained oxygen and nutrients for its survival and also throw the waste products into this river of water. These waste products if not removed becomes toxic for the cell.; http://drsangita.blogspot.com
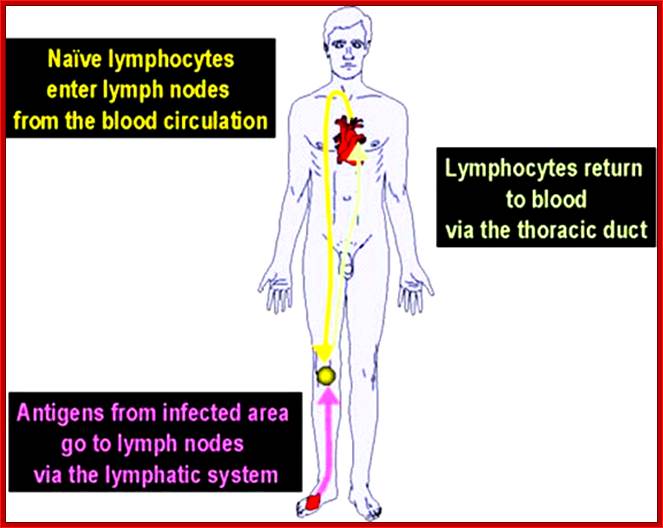
Circulation lymph cells
http://anatomyzone.com
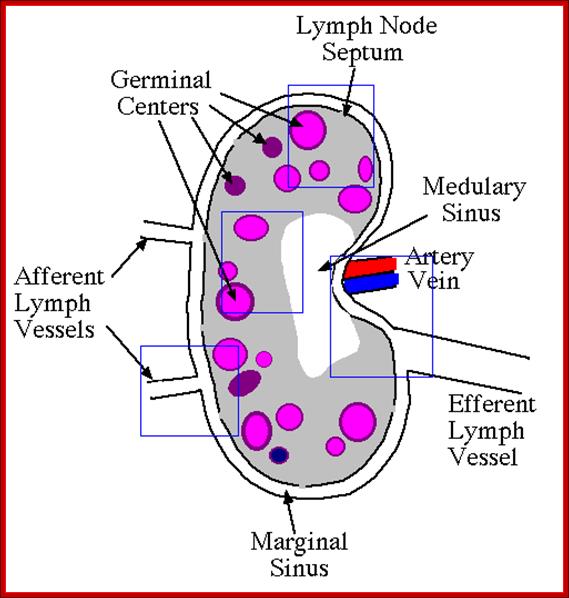
Lymph Node;http://www2.nau.edu
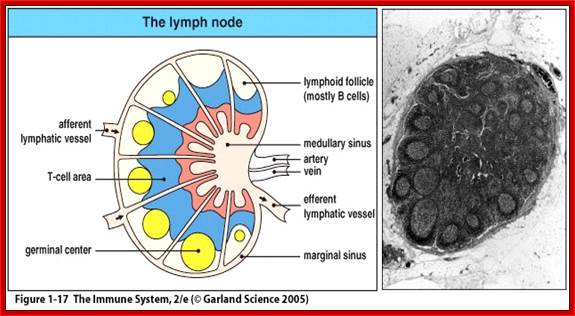
https://www.studyblue.comhttp://humananatomychart.us
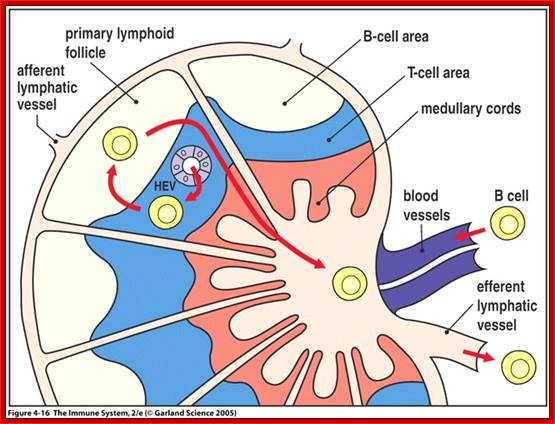
Lindsea et al; http://journal.frontiersin.org
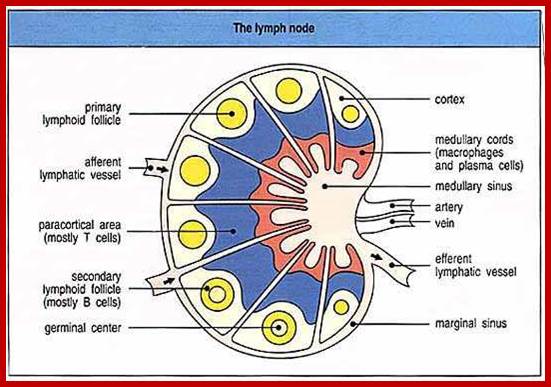
http://www.dartmouth.edu;Lymph Node
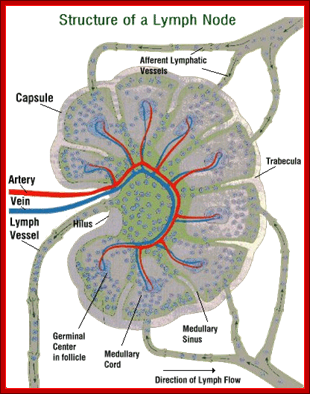
This picture shows the regions of the lymph node where the blood stream (arteries and veins) anter a typical lymph node. For the lymphatic system to function properly in its defensive role, the lymph nodes must be able to "dump" their leukocytes (infection fighting cells) quickly into the general blood stream. It is important to note that white blood cells are not produced in the Lymph nodes initially, only stored there. In the event of a serious infection (a pathogenic virus for example), the lymph nodes often become very swollen. This swelling represents the explosive multiplication of leukocyte numbers in the lymph node's honeycomb of connective tissue. http://www.acm.uiuc.edu/
Dendrite Cells.
Dendrite cells form a part of immune system.� They are responsible for presenting antigen to lymphocytes. Dendritic cells are present in small quantities in tissues that are in contact with the external environment, mainly the skin (where there is a specialized dendritic cell type called Langerhans cells) and the inner lining of the nose, lungs, stomach and intestines. They can also be found in an immature state in the blood. Once activated, they migrate to the lymphoid tissues where they interact with T cells and B cells to initiate and shape the adaptive immune response. At certain development stages they grow branched projections, the ''dendrites'', that give the cell its name. However, these do not have any special relation with neurons, which also possess similar appendages. Immature dendritic cells are also called veiled cells, in which case they possess large cytoplasmic 'veils' rather than dendrites.
Dendritic cells were first described by Paul Langerhans (Langerhans cells) in the late nineteenth century. It wasn't until 1973, however, that the term "dendritic cells" was coined by Ralph M. Steinman and Zanvil A. Cohn.. In 2007 Steinman was awarded the Albert Lasker Award for Basic Medical Research for his discovery.
|
Name |
Description |
Secretion |
Toll-like receptors |
|
Myeloid dendritic cell (mDC) |
Most similar to monocytes. mDC are
made up of at least two subsets: |
IL-12 |
TLR 2, TLR 4 |
|
Plasmacytoid dendritic cell (pDC) |
Look like plasma cells, but have certain characteristics similar to myeloid dendritic cells. |
Can produce high amounts of interferon-alpha and thus became known as IPC (interferon-producing cells) before their dendritic cell nature was revealed. |
TLR 7, TLR 9 |
The markers BDCA-2, BDCA-3, and BDCA-4 can be used to discriminate among the types.
Lymphoid and myeloid DCs evolve from lymphoid or myeloid precursors respectively and thus are of hematopoietic origin. By contrast, follicular dendritic cells (FDC) are probably of mesenchymal rather than hematopoietic origin and do not express MHC class II, but are so named because they are located in lymphoid follicles and have long "dendritic" processes.
Dendritic cells are derived from hemopoietic bone marrow progenitor cells. These progenitor cells initially transform into immature dendritic cells. These cells are characterized by high endocytic activity and low T-cell activation potential. Immature dendritic cells constantly sample the surrounding environment for pathogens such as viruses and bacteria. This is done through pattern recognition receptors (PRRs) such as the toll-like receptors (TLRs). TLRs recognize specific chemical signatures found on subsets of pathogens. Immature dendritic cells may also phagocytose small quantities of membrane from live own cells, in a process called nibbling. Once they have come into contact with a presentable antigen, they become activated into mature dendritic cells and begin to migrate to the lymph node
The dendritic cells are constantly in communication with other cells in the body. This communication can take the form of direct cell-to-cell contact based on the interaction of cell-surface proteins. An example of this includes the interaction of the receptor B7 of the dendritic cell with CD28 present on the lymphocyte. However, the cell-cell interaction can also take place at a distance via cytokines.
For example, stimulating dendritic cells ''in vivo'' with microbial extracts causes the dendritic cells to rapidly begin producing IL-12. IL-12 is a signal that helps send naive CD4 T cells towards a Th1 phenotype.
The ultimate consequence is priming and activation of the immune system for attack against the antigens which the dendritic cell presents on its surface. However, there are differences in the cytokines produced depending on the type of dendritic cell. The lymphoid DC has the ability to produce huge amounts of type-1 IFN's, which recruit more activated macrophage to allow phagocytosis (from News Medical)
Checkpoints of Dendritic-Cell Immunology, Nature Reviews
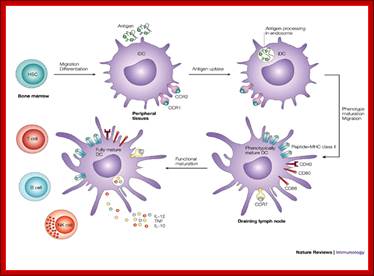
Checkpoints of dendritic-cell immunology; Holger Hackstein & Angus W. Thomson; http://www.nature.com
Haematopoietic stem cells (HSCs) differentiate into immature dendritic cells (iDCs) that are recruited to peripheral tissues, where they continuously internalize antigens that can be processed by an endosomal, MHC class-II-restricted pathway. After antigen capture and depending on the nature of the antigen, DCs migrate to the draining lymphoid tissue and mature phenotypically, upregulating the expression of CD40, CD80, CD86, MHC class II molecules and CC-chemokine receptor 7 (CCR7). In the draining lymphoid tissue, they present peptide�MHC class II complexes on the cell surface, interact with antigen-specific lymphocytes and mature functionally, activating T cells, B cells and natural killer (NK) cells and producing pro-inflammatory cytokines, such as interleukin-12 (IL-12) and tumour-necrosis factor (TNF) Dendritic cells: emerging pharmacological targets of immunosuppressive drugs Holger Hackstein & Angus W. Thomson
Mast Cells:
Mast cells were first described by Paul Ehrlich in his 1878 doctoral thesis on the basis of their unique staining characteristics and large granules. These granules also led him to the mistaken belief that they existed to nourish the surrounding tissue, and he named them "Mastzellen" (from the German: Mast, "fattening" as of animals). They are now considered to be part of the immune system. Mast cells close to basophil granulocytes in blood,the similarities between mast cells and basophils have led many to speculate that mast cells are basophils that have "homed in" on tissues. However, current evidence suggests that they are generated by different precursor cells in the bone marrow.
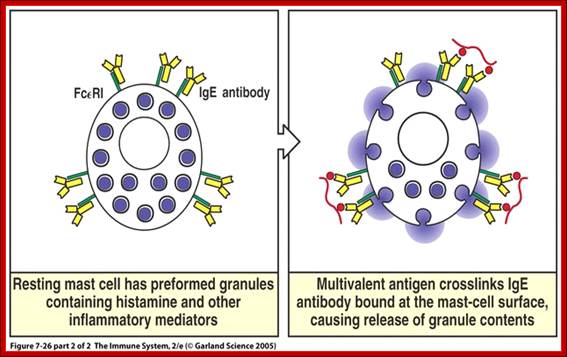
Mast cells
Physiology
Mast cells play a key role in the inflammatory process. When activated, a mast cell rapidly releases its characteristic granules and various hormonal mediators into the interstitium. Mast cells can be stimulated to degranulate by direct injury (e.g. physical or chemical [such as opioids, alcohols, and certain antibiotics such as polymyxins]), cross-linking of Immunoglobulin E (IgE) receptors, or by activated complement proteins.[2]
Mast cells express a high-affinity receptor (FcεRI) for the Fc region of IgE, the least-abundant member of the antibodies. This receptor is of such high affinity that binding of IgE molecules is essentially irreversible. As a result, mast cells are coated with IgE. IgE is produced by plasma cells (the antibody-producing cells of the immune system). IgE molecules, like all antibodies, are specific to one particular antigen
A mast cell is part of a group of cells called leucocytes. Leucocytes are white blood cells and are found in the blood plasma with erythrocytes, red blood cells. Mast cells have immunological functions, or they are part of the immune system. They form part of an early warning system. When stimulated, they release chemicals that signal either injury or infection and cause an inflammation in the area.
The chemicals that are produced by a mast cell are called mediators. Two common mediators are histamine and heparin. Histamine, the most important chemical mediator, causes capillary walls to become more permeable, or let substances through. Heparin prevents blood from clotting to allow blood to flow to the area of infection or injury. Mast cells play an important role in allergic reactions because of their ability to produce and release histamine.
During an immune response, a mast cell is stimulated by a specific type of antibody, called IgE or immunoglobulin E. Antibodies are grouped into classes based on a chemical chain, or tail, attached to them. There are five classes of antibodies based on the specific amino acid sequence of the chains, A, D, E, G and M. All antibodies are called immunoglobulins, so they are referred to as IgA, IgD, etc.
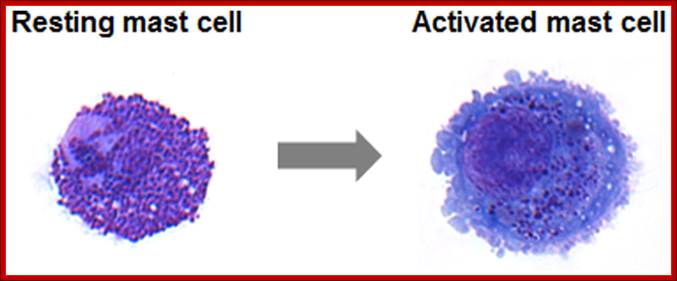
May�Gr�nwald/Giemsa stain of a resting human intestinal mast cell and a mast cell following activation induced degranulation. Note the increase in size and loss of granule staining (Figure from Lorentz et al., 2012).;http://journal.frontiersin.org/
Mast cells.
Mast Cell; http://www.clker.com/
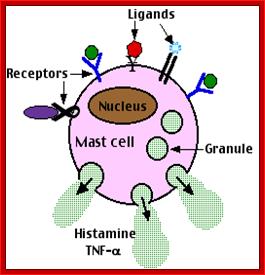
Activated mast cell releases histamines; http://users.rcn.com/kimall.ma.ultanet
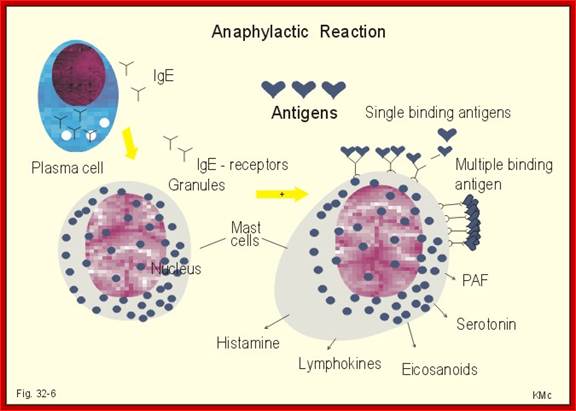
Anaphylactic reaction as it occurs in mast cells and basophils.; http://www.zuniv.net/
The molecules thus released into the extracellular environment include:
� preformed mediators (from the granules):
� serine proteases, such as tryptase
� proteoglycans, mainly heparin (active as anticoagulant)
� newly formed lipid mediators (eicosanoids):
� Eosinophil chemotactic factor
Macrophages
Macrophage: A type of white blood cell that ingests foreign material. Macrophages are key players in the immune response to foreign invaders of the body, such as infectious microorganisms. They are normally found in the liver, spleen, and connective tissues of the body.
The term "macrophage" conjures images of a hungry white blood cell gobbling invading bacteria. However, macrophages do much more than that: Not only do they act as antimicrobial warriors, they also play critical roles in immune regulation and wound-healing. They can respond to a variety of cellular signals and change their physiology in response to local cues.
Monocyte Differentiation
Macrophages exist in nearly all tissues and are produced when white blood cells called monocytes leave the blood and differentiate in a tissue-specific manner. The type of macrophage that results from monocyte differentiation depends on the type(s) of cytokines that these cells encounter. Cytokines are proteins produced by immune cells that can influence cell behavior and affect interactions between cells. For example, macrophages that battle microbial invaders arise in response to interferon-γ, a cytokine that is produced during a cellular immune response involving helper T-cells and the factors they produce. These macrophages are considered to be "classically activated."
However, when monocytes differentiate in response to stimuli such as prostaglandins or glucocorticoids, the resulting macrophages will assume a "regulatory" phenotype. Alternately, wound-healing macrophages arise when monocytes differentiate in response to interleukin-4, a cytokine which is released during tissue injury.
According to Dr. Mosser, macrophages can change their physiology and switch types. For example, in healthy, non-obese people, macrophages in fat tend to function as wound-healing macrophages. They are also thought to maintain insulin sensitivity in adipose cells. However, should an individual become obese, macrophages in fat will instead promote inflammation and cause the adipose cells to become resistant to insulin.
Immune Regulation
Immune-regulating macrophages produce high levels of the cytokine interleukin-10, which helps suppress the body's immune response. Suppressing an immune response may seem counter-intuitive, but in the later stages of immunity it comes in handy because it limits inflammation.
According to Dr. Mosser, immune-regulating macrophages may hold the key to developing treatments for autoimmune diseases such as multiple sclerosis or rheumatoid arthritis. The focus of new research is on reprogramming the macrophages to assume a regulatory phenotype and prevent autoimmunity, he said.
There is broad potential for exploiting different stages of macrophage activation, Dr. Mosser added. "It might be possible to manipulate macrophages to make better vaccines, prevent immunosuppression, or develop novel therapeutics that promote anti-inflammatory immune responses."
Wound Healing
The release of interleukin-4 in response to tissue injury not only results in macrophages that specialize in wound-healing, it allows the macrophages to convert arginine to ornithine, which is a precursor of polyamines and collagen. Both polyamines and collagen are instrumental to the formation and maintenance of extracellular matrix, the material between cells that gives them structural support.
Certain harmful microbes, such as the tropical parasite Leishmania spp., can exploit wound-healing macrophages, said Dr. Mosser. "If you have a macrophage whose job it is to promote wound-healing, that macrophage will not be capable of killing microbes," he said. "The microbe can enter the macrophage and survive inside, which is not good for the human host."
Infection with Leishmania spp. causes leishmaniasis, which is characterized by skin sores and ulcers and can enlarge the spleen, damage the liver, and cause anemia. At worst, it can decrease immunity and leave victims vulnerable to potentially fatal opportunistic infections. Survivors can suffer from immune reconstitution inflammatory syndrome, in which their recovering immune systems go overboard in response to infection and create an inflammatory response that makes symptoms even worse. Understanding how Leishmania exploits macrophages has led to a better understanding of how macrophages function in health and disease. It has also stressed the importance of treating infections early, before the bugs can wreak havoc on the immune system.
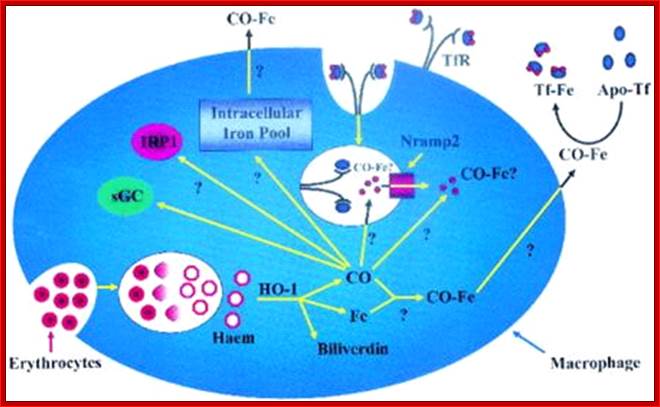
Macrophage consuming RBC; http://www.michigankoi.com/
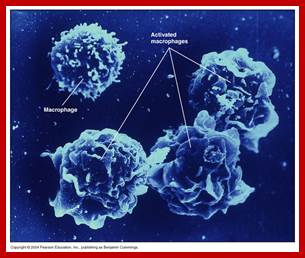
https://bsi.immunology.org
Caption: Macrophage. Coloured scanning electron micrograph (SEM) of a macrophage white blood cell at the site of a skin wound. Macrophages are cells of the body's immune system. As well as fighting off micro-organisms, they play an important role in wound healing. Macrophages arrive at a wound after other immune cells have started eliminating any micro-organisms. They release chemicals that attract other cells to the area, and that start the reconstruction of tissues. They stimulate fibroblasts, cells that produce collagen, and initiate the growth of vascular tissue.
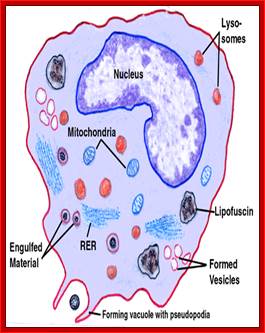
http://web.stanford.edu/
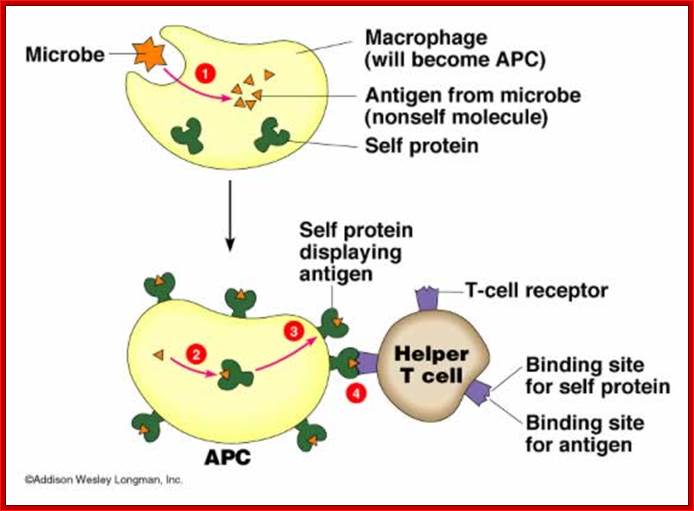
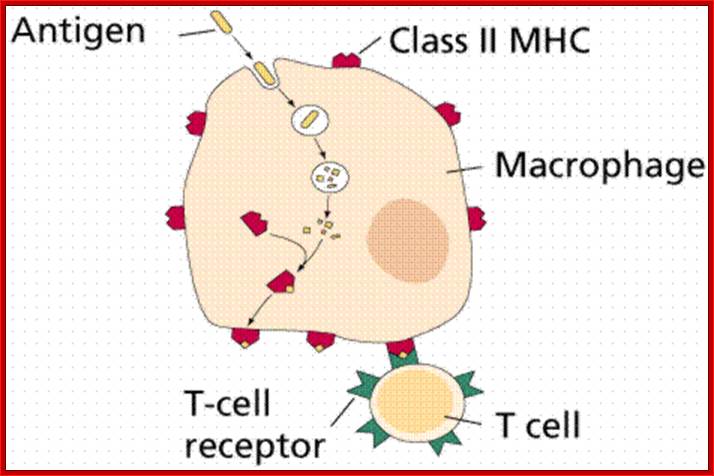
Top Fig,Lecture Notes on Immune system; http://www.anselm.edu/;� Bottom Fig.Lymphatic system and Immunity; www2.estrellamountain.ed
Macrophages are very common. These phagocytic wandering cells are one of the major lines of defense against infection, and can be found in most loose CT. You should be able to find macrophages on your own slide 27, in the loose CT of the inflamed salivary gland, from which the image at left comes. Scan the slide at low power looking for brownish pigment. This is hemosiderin and/or lipofuscin, in macrophages that have migrated into the area.
These cells contain brown granules of undigested cellular debris and blood cells. Macrophages come in to clean up debris, hemorrhage, and dead cells. Their arrival and activity are normal parts of the sequence of events in the process inflammation and resolution. Macrophages are derived from circulating blood monocytes (see Exercise 6). There is considerable evidence to indicate that the monocyte isn't a "blood cell" at all: it just uses the blood as a transport mechanism to get where it has to go, then leaves the vascular system for the connective tissue space, where it differentiates into a macrophage.
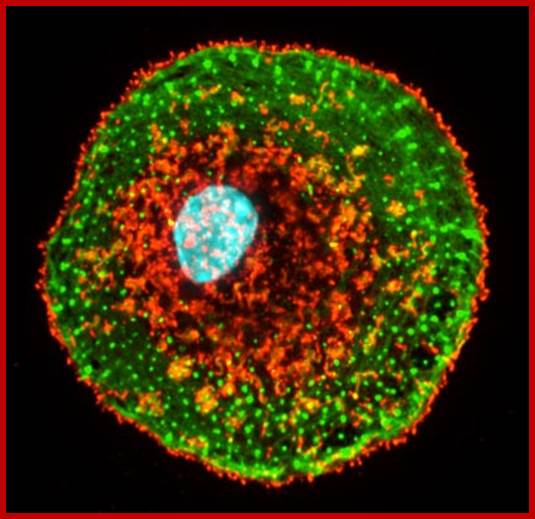
Immunofluorescence of human primary macrophages stained for F-actin using Alexa Fluor488-phalloidin (green), for DNA using DAPI (light blue) and for CD9 using an mouse anti-human CD9 primary antibody followed by a donkey anti-mouse Cy3-labeled secondary. CD9 is a member of the tetraspanin family. Research Group of Dr Sergio Grinstein, Hospital for Sick Children Research Institute; http://cellularimaging.perkinelmer.com
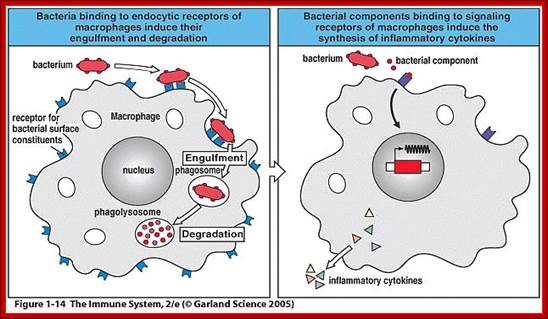
Garland science 2005
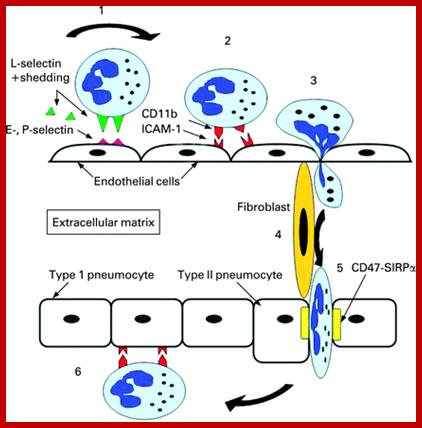
(1) Transient tethering of the neutrophil to the endothelial surface is mediated by selectins (green and pink) and results in rolling adhesion. (2) Strong adhesion to the vessel wall is mediated by activated β2 integrins (red). (3) Migration between the endothelial cells is facilitated by chemokines and chemoattractants. (4) Migration through the extracellular matrix occurs along fibroblasts. (5) Migration through the alveolar epithelial cells uses CD47-signal regulatory protein α (SIRPα) (yellow). (6) Tethering to the apical surface of the alveolar epithelial cells via β2 integrins and ICAM-1 (red). ICAM-1, intercellular adhesion molecule 1.;http://thorax.bmj.com
����������� Antigen presenting cells:
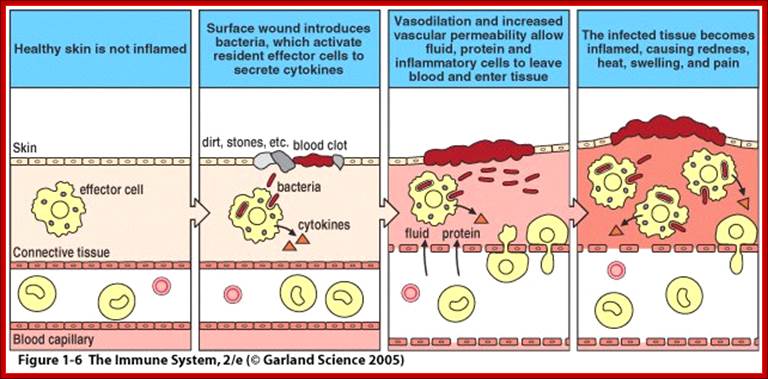 Garland
Science 2005
Garland
Science 2005
Cells� movement causes inflammation to injury site:
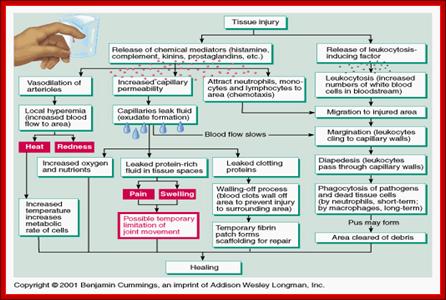
Neutrophils
� This is the most common granulocyte, accounting for more than half of the total amount of white blood cells. They have incredibly short life spans, remaining in the blood for only about 12 hours. These cells work hard to perform phagocytosis, the engulfing of and destroying of debris and pathogens. After battling an infection, dead neutrophils are left behind with a mixture of fluid and other cell parts that is called pus. Neutrophils are essential and a deficiency of them is considered life threatening.
Basophils
� Basophils are usually present in much smaller numbers than neutrophils. They account for less than 1% of the total white blood cells. They are known to play a role in releasing histamine during a response to inflammation. They also release the anticoagulant heparin. Basophils are usually found in areas such as the lungs and the liver, where there is a large volume of blood, and it's possible that the heparin they release helps prevent tiny blood clots from forming. �www.labvision.com
Eosinophils
� Eosinophils are responsible for 1-3% of leukocytes. While much is not known about them, it is clear that they are involved in the body's response to inflammation. Eosinophils release chemicals that can destroy pathogens. A person who is in the midst of an allergic reaction will have an increased number of eosinophils in their bloodstream, as will a person who is fighting an infection of a parasitic nature.
Monocytes
� Monocytes, like neutrophils, can perform phagocytosis. They only account for 3 -- 8 % of all white blood cells, yet they are more efficient at destroying pathogens. These cells change into macrophages, which are able to leave the bloodstream and enter body tissue. Monocytes then patrol the body looking for pathogens and debris to clean up. Macrophages are common in the mucus membranes and under the skin, where they can be readily available to fight anything that invades the body through a small tear or scratch. These cells provide another service to the body; they phagocytose old red blood cells, helping the bloodstream remain healthy.
Read more: Structure &
Function of White Blood Cells: | eHow.com http://www.ehow.com/
Lymphocytes:
from MedFriendly
Lymphocytes are considered to be of the lymphoid lineage as opposed to other lineages of blood cells such as the myeloid lineage and the erythroid lineage.� Lymphopoiesis is now used interchangeably with the term "lymphocytopoiesis" - the making of lymphocytes - but other sources may distinguish between the two, stating that "lymphopoiesis" additionally refers to creating lymphatic tissue, while "lymphocytopoiesis" refers only to the creation of cells in that tissue. It is rare now for lymphopoiesis to refer to the creation of lymphatic tissues.
A lymphocyte is a type of white blood cell present in the blood. White blood cells help protect the body against diseases and fight infections. When the general defense systems of the body have been penetrated by dangerous invading microorganisms, lymphocytes help provide a specific response to attack the invading organisms. A microorganism is a tiny organism made of one cell that is usually too small to be seen without using a microscope. Lymphocytes help to protect the body against tumors (tissues that grow more rapidly than normal). However, lymphocytes can also cause the rejection of tissues during organ transplants because they interpret these tissues as foreign invaders. Lymphocytes
� Lymphocytes make up between 25 -- 38 % of all leukocytes. Lymphocytes play a huge part in the body's immune system. There are two subdivisions of lymphocytes, B-lymphocytes and T-lymphocytes. B-lymphocytes are created In the bone marrow, while T-lymphocytes are generated from the thymus gland. The main function of these cells is to create and release antibodies and to protect the body from cancer cells.
What are some other characteristics of Lymphocytes?
Lymphocytes are small white
blood cells, usually 7 to 8 micrometers in length. A micrometer is a very small
unit of length that measures one millionth of a meter. A meter is approximately
39 inches (slightly more than 3 feet). Larger forms of lymphocytes are usually
about 10 to 20 micrometers in length.
The nucleus (central structure) of a lymphocyte is made of large
groupings of thin threads known as chromatin. The nucleus of a lymphocyte
stains dark purple/blue when exposed to a stain known as Wright's stain. You
can see what this looks like below. As you can see, the nucleus is usually
round but can be slightly indented. Also, the nucleus is surrounded by a small
amount of light blue cytoplasm (a gel-like substance that fills up a cell).
Unlike other types of white blood cells, such as basophils and eosinophils, the cytoplasm of lymphocytes usually do not contain large,
rough-looking, grain-like particles. However, larger forms of lymphocytes may
have a lot of cytoplasm that contain several bright reddish/purplish,
rough-looking, grain-like particles. Unlike some other types of cells, the
granules of lymphocytes do not turn a blue color when exposed to certain types
of chemical used in laboratory tests.
������� Current information indicates that regardless of the variability of organized lymphoid tissues and the sometimes subtle diversity of immune responses in evolutionarily distant forms, the small lymphocyte has been maintained both morphologically and as an effector of immune reactivities throughout vertebrate phylogeny. Moreover, the basic properties of the mammalian lymphocytes�the discriminatory recognition of nonself plus the amplification steps that follow such a recognition event appear to be common to lymphocytes from all vertebrates. Although examples of lymphocyte heterogeneity and cellular cooperation have been reported for representative elasmobranchs, teleosts, and amphibians, the extent and nature of lymphocyte diversity remain as major unsolved problems currently being investigated by immunologists. � 1975 by the American Society of Zoologists- NICHOLAS COHEN.
What is a T cell?;
Scanning electron micrograph of a human T cell; http://www.washington.edu/
T cell is a kind of lymphocyte (WBC),plays an important role in the immune system.� T lymphocytes are formed in the Thymus gland and react highly and specifically against the particular type of Antigen that initiated their development.� T-lymphocytes account for more than four-fifth of lymphocytes; http://www.daviddarling.info/
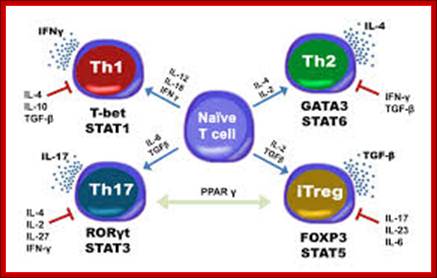
Schematic representation of the cytokines and transcription factors controlling CD4+ T cell differentiation. Our CD4+ T cell differentiation model is firmly grounded on experimental observations and reproduces four CD4+ T cell phenotypes upon external stimulation with appropriate cytokine combinations, as well as representing the crosstalk between phenotypes, exhibiting inhibitory trends. http://www.modelingimmunity.org/
T cells
(also known as T lymphocytes) are types of lymphocyte that circulate through
the thymus gland and have turned into cells known as thymocytes (cells that
have developed in the thymus gland). The thymus gland is an organ located in the upper part of the chest
and is very important in producing substances that protect the body against
disease. When thymocytes are exposed to antigens (substances in the body, such
as those present on the surface of bacteria, that can produce a defensive
reaction by the body), they rapidly divide and produce large numbers of new T
cells that are sensitive to that type of antigen. More than 80% of lymphocytes
in the circulating blood are T cells.
Reprogramming immune system cells to produce Natural Killer cells for cancer cells; http://www.sanger.ac.uk/
Cytotoxic- T cell
In one of those bizarre twists of logic, cytotoxic T lymphocytes were so named because they�re T lymphocytes that are cytotoxic. Is that all they are?
Cytotoxicity is relatively easy to measure � there are straightforward ways to measure cell death, and it can be a nice, binary, black/white distinction. If you take lymphocytes from a mouse (or a person) that was previously infected with a virus, and you mix those lymphocytes with cells infected with the same virus, the infected cells will be killed. If you look at the surface markers of the cells responsible for the killing, you can narrow it down to T cells (i.e. with the T cell receptor) that have the CD8 surface marker
Natural Killer Cell attacks a tumor cell.; Source: unm.edu,; http://eliminatecancer.hubpages.com/
T lymphocytes and cancer cell, SEM; Credit: Caption: T lymphocytes and cancer cell. Coloured scanning electron micrograph (SEM) of T lymphocyte cells (pink) attached to a cancer cell. T lymphocytes are a type of white blood cell that recognise a specific site (antigen) on the surface of cancer cells or pathogens and bind to it. Some T lymphocytes then signal for other immune system cells to eliminate the cell. The genetic changes that cause a cell to become cancerous lead to the presentation of tumour antigens on the cell's surface. Magnification: x2300 when printed at 10 centimetres wide; STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY.
There are two main groups of T cells. One group of T cells are called "killer cells" (also known as cytotoxic T cells) because they produce chemical substances known as lymphokines that are essential in helping the B cells (see next section) destroy foreign substances. Like B cells (as is described in the next section), T cells are sensitized and stimulated to respond to certain antigens present on invading microorganisms or abnormal cells. Another group of T cells are called helper T cells. Helper T cells assist the killer T cells in performing their activities and help protect the body against diseases in other ways.
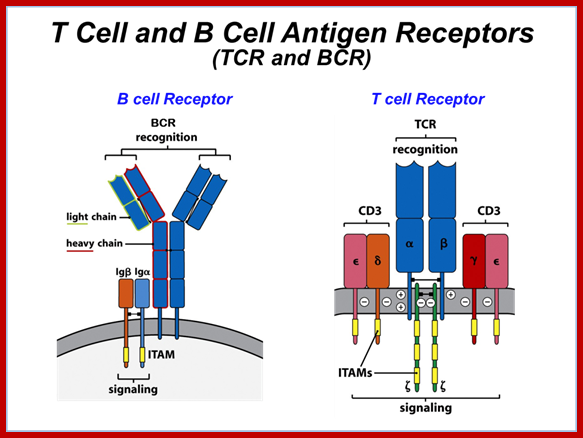
Immunological Aspects of Organ Transplantation http://drjosephtm.blogspot.com
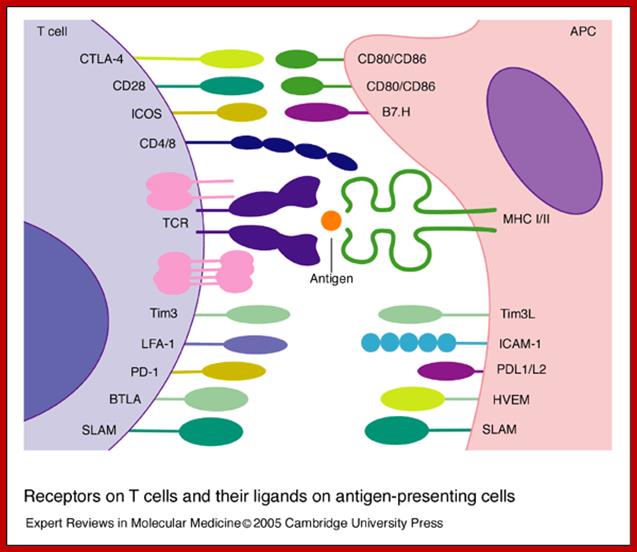
Beverley Wilkinson, et alhttp://journals.cambridge.org; http://www.expertreviews.org/
In addition to specific antigen recognition through the TCR, T-cell activation is regulated through a balance of positive and negative signals provided by co-stimulatory receptors. These surface proteins are typically members of either the TNF receptor or B7 superfamilies. Agonistic antibodies directed against activating co-stimulatory molecules and blocking antibodies against negative co-stimulatory molecules may enhance T-cell stimulation to promote tumour destruction.
�T cells also appear to play an�� important role in the body's response to the
spreading of cancer cells. Cancer is a group of diseases in which symptoms are due to an abnormal and excessive growth of cells in one of
the body organs or tissues. The process of the T cells protecting the body is
known as cellular or cell-mediated immunity.
T cells reproduce through a process known as mitosis, in which the cells split
in two. In mitosis, each cell contains an exact copy of the chromosomes in the
original cell. Chromosomes are structures in a person's cells that contain
proteins and a substance known as DNA (an abbreviation for deoxyribonucleic
acid). DNA is a chain of many connected genes. Genes are units of material
contained in a person's cells that contain coded instructions as for how
certain bodily characteristics (such as eye colour) will develop.
Lymphopoiesis for T cells;
T cells are formed in bone marrow then migrate to the cortex of the thymus to undergo maturation in an antigen-free environment for about one week where a mere 2-4% of the T cells succeed. The remaining 96-98% of T cells die by apoptosis and are phagocytosed by macrophages in the thymus. So many thymocytes (T cells) die during the maturation process because there is intensive screening to make sure each thymocyte has the ability to recognize self-peptide:self-MHC complex �and for self tolerance. The apoptosed thymocyte dies a willing and noble death and it is quickly recycled.
Upon maturity, there are several forms of thymocytes including [11]
� T-helper (needed for activation of other cells such as B cells and macrophages),
� T-cytotoxic (which kill virally-infected cells),
� T-memory (T cells that remember antigens previously encountered), and
� T-suppressor cells (which moderate the immune response of other leukocytes). Also called T-regulatory Cell, or just �Treg(s)�, to be cool.
Types of Cells:
T Helper Cell.
T helper cell (TH cells) assist other white blood cells in immunologic processes, including maturation of B cells into plasma cells and memory B cells, and activation of cytotoxic T cells and macrophages. These cells are also known as CD4+ T cells because they express the CD4 protein on their surface. Helper T cells become activated when they are presented with peptide antigens by MHC class II molecules, which are expressed on the surface of antigen presenting cells (APCs). Once activated, they divide rapidly and secrete small proteins called cytokines that regulate or assist in the active immune response. These cells can differentiate into one of several subtypes, including TH1, TH2, TH3, TH17, or TFH, which secrete different cytokines to facilitate a different type of immune response. Signalling from the APC directs T cells into particular subtypes.
Cytotoxic Cell
Cytotoxic T cells (TC cells, or CTLs) destroy virally infected cells and tumor cells, and are also implicated in transplant rejection. These cells are also known as CD8+ T cells since they express the CD8 glycoprotein at their surface. These cells recognize their targets by binding to antigen associated with MHC class I, which is present on the surface of nearly every cell of the body. Through IL-10, adenosine and other molecules secreted by regulatory T cells, the CD8+ cells can be inactivated to an anergic state, which prevent autoimmune diseases such as experimental autoimmune encephalomyelitis.
Memory Cell
Memory T cells are a subset of antigen-specific T cells that persist long-term after an infection has resolved. They quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen, thus providing the immune system with "memory" against past infections. Memory T cells comprise two subtypes: central memory T cells (TCM cells) and effector memory T cells (TEM cells). Memory cells may be either CD4+ or CD8+. Memory T cells typically express the cell surface protein CD45RO.
Regulatory Cell
Regulatory T cells (Treg cells), formerly known as suppressor T cells, are crucial for the maintenance of immunological tolerance. Their major role is to shut down T cell-mediated immunity toward the end of an immune reaction and to suppress auto-reactive T cells that escaped the process of negative selection in the thymus.
Two major classes of CD4+ Treg cells have been described � naturally occurring Treg cells and adaptive Treg cells.
Naturally occurring Treg cells (also known as CD4+CD25+FoxP3+ Treg cells) arise in the thymus and have been linked to interactions between developing T cells with both myeloid (CD11c+) and plasmacytoid (CD123+) dendritic cells that have been activated with TSLP. Naturally occurring Treg cells can be distinguished from other T cells by the presence of an intracellular molecule called FoxP3. Mutations of the FOXP3 gene can prevent regulatory T cell development, causing the fatal autoimmune disease IPEX.
Adaptive Treg cells (also known as Tr1 cells or Th3 cells) may originate during a normal immune response.
Natural killer Cell
Natural killer T cells (NKT cells � not to be confused with natural killer cells) bridge the adaptive immune system with the innate immune system. Unlike conventional T cells that recognize peptide antigens presented by major histocompatibility complex (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called CD1d. Once activated, these cells can perform functions ascribed to both Th and Tc cells (i.e., cytokine production and release of cytolytic/cell killing molecules). They are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses.
Γδ T Cells
γδ T cells (gamma delta T cells) represent a small subset of T cells that possess a distinct T cell receptor (TCR) on their surface. A majority of T cells have a TCR composed of two glycoprotein chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common (2% of total T cells) than the αβ T cells, but are found at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs). The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on antigen presenting cells. Some murine γδ T cells recognize MHC class IB molecules though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of non-peptidic phosphorylated isoprenoid precursors, collectively named phosphoantigens. Phosphoantigens are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP). Many microbes produce the highly active compound hydroxy-DMAPP (HMB-PP) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and aminobisphosphonates, which up-regulate endogenous IPP/DMAPP
All T cells originate from haematopoietic stem cells in the bone marrow. Haematopoietic progenitors derived from haematopoietic stem cells populate the thymus and expand by cell division to generate a large population of immature thymocytes. The earliest thymocytes express neither CD4 nor CD8, and are therefore classed as double-negative (CD4-CD8-) cells. As they progress through their development they become double-positive thymocytes (CD4+CD8+), and finally mature to single-positive (CD4+CD8- or CD4-CD8+) thymocytes that are then released from the thymus to peripheral tissues.
About 98% of thymocytes die during the development processes in the thymus by failing either positive selection or negative selection, whereas the other 2% survive and leave the thymus to become mature immunocompetent T cells.
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3% a year throughout middle age, there is a corresponding fall in the thymic production of naive T cells, leaving peripheral T cell expansion to play a greater role in protecting older subjects.
What is a B cell?
B cell is a lymphocyte. Immature B cells are produced in the bone marrow of most mammals. Rabbits are an exception; their B cells develop in the appendix-sacculus rotundus. After reaching the IgM+ immature stage in the bone marrow, these immature B cells migrate to secondary lymphoid tissues (such as the spleen, lymph nodes, Peyer's patches, etc.) where they are called transitional B cells, and some of these cells differentiate into mature B lymphocytes.
B cell development occurs through several stages, each stage representing a change in the genome content at the antibody loci. An antibody is composed of two identical light (L) and two identical heavy (H) chains, and the genes specifying them are found in the 'V' (Variable) region and the 'C' (Constant) region. In the heavy-chain 'V' region there are three segments; V, D and J, which recombine randomly, in a process called VDJ recombination, to produce a unique variable domain in the immunoglobulin of each individual B cell. Similar rearrangements occur for light-chain 'V' region except there are only two segments involved; V and J. The list below describes the process of immunoglobulin formation at the different stages of B cell development. When the B cell fails in any step of the maturation process, it will die by a mechanism called apoptosis, here called clonal deletion. �B cells are continuously produced in the bone marrow. When the B cell receptor, on the surface of the cell matches the detected antigens present in the body, the B cell proliferates and secretes a free form of those receptors (antibodies) with identical binding sites as the ones on the original cell surface. After activation, the cell proliferates and B memory cells would form to recognise the same antigen. This information would then be used as a part of the adaptive immune system for a more efficient and more powerful immune response for future encounters with that antigen.
B cell membrane receptors evolve and change throughout the B cell life span. �TACI, BCMA and BAFF-R are present on both immature B cells and mature B cells. All of these 3 receptors may be inhibited by Belimumab. CD20 is expressed on all stages of B cell development except the first and last; it is present from pre-B cells through memory cells, but not on either pre-pro-B cells or plasma cells
B cells (also known as B lymphocytes) are types of lymphocytes
that circulates in the blood in an immature (not fully developed) form. About
10% of lympocytes that circulate in the blood are B cells. B cells produce
proteins known as antibodies that are then inserted into the area that
immediately surround the cytoplasm (a gooey substance that fills up a cell).
Antibodies attach to foreign proteins in the body known as antigens that are
found on the surface on certain microorganisms. A microorganism is a tiny
organism made of one cell that is usually too small to be seen without using a
microscope.
As an example of the above, some antibodies help fight against bacteria because
bacteria commonly contain many antigens. When the antibodies attach to the
antigens on the microorganism, this starts a process that lead to the death of
the microorganism. This process of the B cells protecting the body is known as
humoral immunity, because the B cells release the antibodies into the fluids
(also known as humors) of the body. B cells also reproduce through a process
known as mitosis (see the last section for a description). When the B cells
divide, each cell has identical copies of antibodies on their surface.
Sometimes an immature B cell is exposed to an antigen that characterizes a single
class of microorganisms. An example of microorganisms would be bacteria. When
the B cell is exposed to such antigens, it becomes activated and travels to the
spleen or the lymph nodes. The spleen is an organ next to the stomach that
helps fight infection and removes and destroys worn-out red blood cells. Lymph
nodes are small egg shaped structures in the body that help fight against
infection. When the B cells reach the spleen and the lymph nodes under such
conditions, they change into plasma cells and memory cells.
 �
�
A suspected plasma cell. Plasma cells are normally detected in tissues rather than in circulation; http://www.3dscience.com/
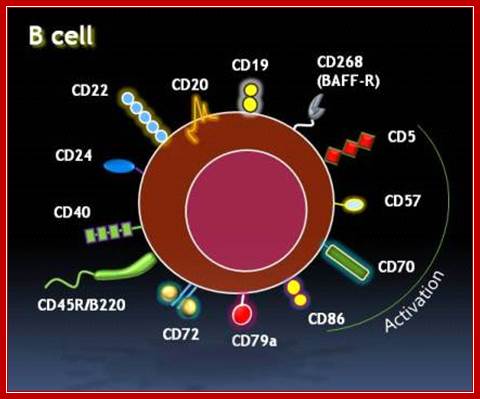
���������������������������� One of the things that is useful to know when looking at the new targeted anti-cancer therapies (TAT) is the phenotype of your MM PC�s. The test that is used to determine this is Flow Cytometry (FCM). FCM specifically determines percents of lymphocytes that contain a particular combination of specific cell surface proteins, IG�s or CD�s or that produce particular cytokines via cytokine staining. When it comes to TAT, phenotypes form the basis for new phenotype dependent treatment for patients with MM. Recall, that phenotype is also referred to as CD�s.B Cell with its membrane interacting proteins; http://myelomacinderella.net/
B Cell Types ; Plasma B cells, Memory B cells, B1 Cells, B2 Cells, Marginal and Follicular B cells-
The plasma cells make and release antibodies. The memory cells do not release antibodies but they "remember" antigens they were exposed to in the past so they can deal with them in a quicker way when exposed to these antigens in the future. When exposed again to one of these antigens the memory cells turn into plasma cells and release antibodies to fight off the antigens. This is how vaccines (preparations given to protect the body against infections) work. That is, vaccines expose the body to certain types of antigens so that the memory cells can be prepared for them if encountered in the future and fight against them quickly. Memory cells can survive in the body for many years.
In the bone marrow
� Pro-B
� Pre-B-I
� Pre-B-II large
� Pre-B-II small
� Imm(ature)
In the spleen
� T1
� T2/T3
� (Marginal Zone (MZ); B-1 ; B-2)
� B-2 further differentiate into: B Cell Types ; Plasma B cells, Memory B cells, B1 Cells, B2 Cells, Marginal and Follicular B cells (?).
B cell�T-cell interactions.
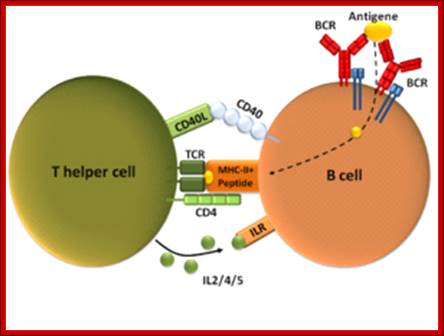
T cell-dependent B cell activation, showing a TH2-cell (left), B cell (right), and several interaction molecules; http://en.wikipedia.org/
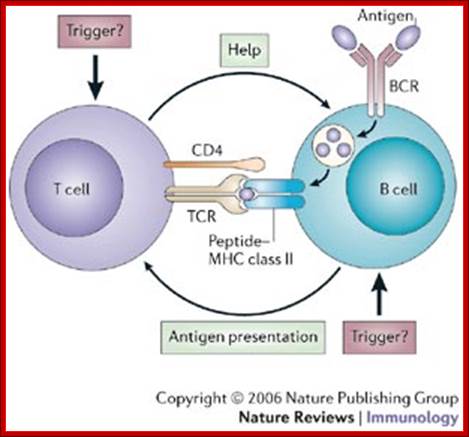
The two-way interaction between B cells and T cells provides the basis for the concept that, in certain autoimmune diseases, an amplification cycle might allow persistent immunopathology to arise from a minor 'trigger' factor. Such a trigger might initiate the cycle through events in either the B-cell or the T-cell compartment, including the stochastic generation of both B-cell receptors (BCRs) and T-cell receptors (TCRs). T cell and B cell interaction, where B cell is activated by T cell, it requires several other interacting molecules. Jonathan C. W. Edwards & Geraldine Cambridge; http://www.nature.com/
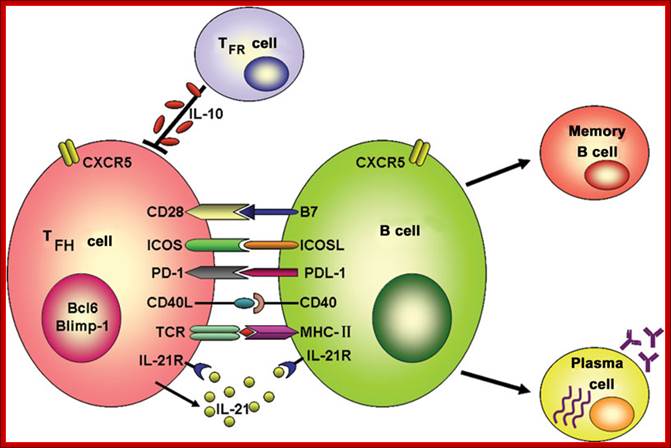
Interaction between TFH and B cells to induce humoral responses. Activated TFH cells upregulate CXCR5 and migrate toward B-cell follicles to form GC. In GC, TFH cells interact with antigen-specific B cells through various molecules such as ICOS�ICOSL, PD-1�PDL-1, CD40�CD40L and IL-21R�IL-21, resulting in the production of memory B and plasma cells. The plasma cells secrete long-lived antibodies to combat infectious agents. However, aberrant TFH cell function leads to the production of autoantibodies that may result in autoimmune pathologies. TFR cells can inhibit the self-reactive B-cell responses such as autoantibody production viasecretion of IL-10. CD40L, CD40 ligand; CXCR5, chemokine receptor-5; GC, germinal center; ICOS, inducible costimulator; PD-1, programmed death-1; TFH, follicular helper T; TFR, follicular T regulatory; Sudhanshu Shekhar and Xi Yanghttp://www.nature.com/������������
B-cell targeting in rheumatoid arthritis and other autoimmune diseases:
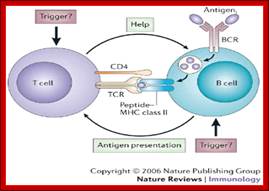
The two-way interaction between B cells and T cells provides the basis for the concept that, in certain autoimmune diseases, an amplification cycle might allow persistent immunopathology to arise from a minor 'trigger' factor. Such a trigger might initiate the cycle through events in either the B-cell or the T-cell compartment, including the stochastic generation of both B-cell receptors (BCRs) and T-cell receptors (TCRs). Jonathan C. W. Edwards & Geraldine Cambridge
What percent of WBCs are Lymphocytes?
Approximately 15% to 40% of white blood cells are lymphocytes. It is important to keep in mind that the ranges mentioned above will be different depending on the machine used to do the blood test. Always use the normal range printed on the lab report to decide what range is normal.
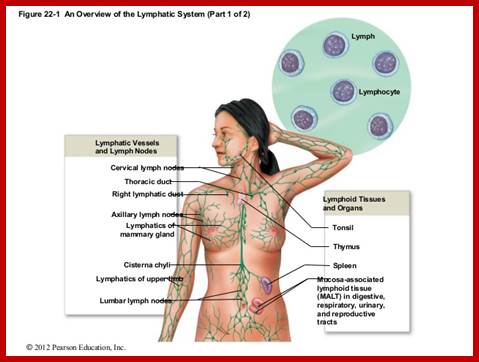
http://www.slideshare.net/
MALT, CALT and GALT Tissues:
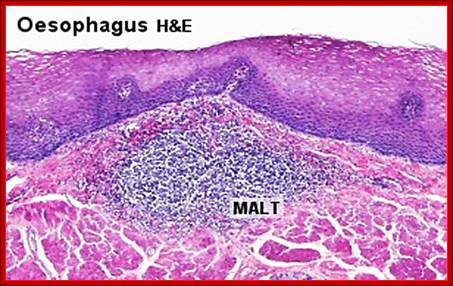
https://cellbiology.med.unsw.edu.au
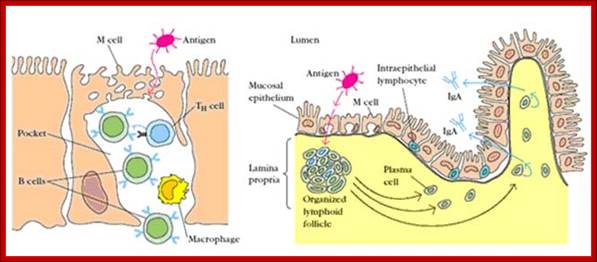
Collectively, the mucosal immune system is estimated to contain as many lymphocytes as all in the rest of the body, and they form a specialized set of cells obeying somewhat different rules. The bulk of the GALT tissue is B cells, organized in large and highly active follicle domains. T cells occupy the areas between follicles. The antigen enters across a specialized epithelium made up of so-called M cells. Although this tissue looks very different from other lymphoid organs, the basic divisions are maintained. Mucose associated Lymphoid Tissue (MALT), Bronchus associated Lymphoid, Gut associated lymphoid tissue, GALT, Cutaneous associated tissue (CALT); http://biosiva.50webs.org/
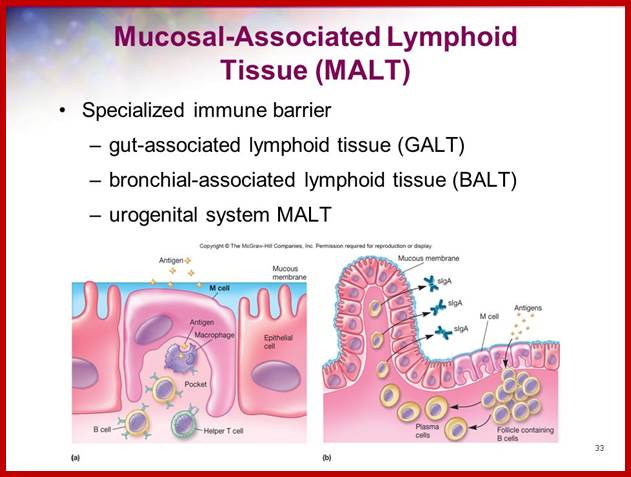
Slideplayer.com
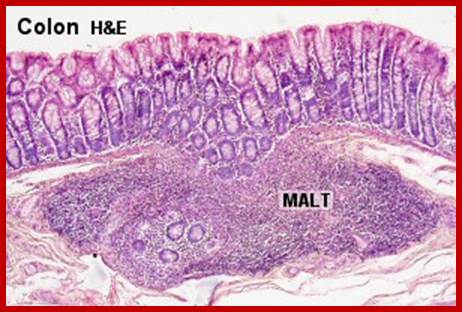
www.lab.anhb.uwa.edu.au

www.slideshare.net
� 1798 Edward Jenner, Smallpox vaccination
� 1862 Ernst Haeckel, Recognition of phagocytosis
� 1877 Paul Erlich, recognition of mast cells
� 1879 Louis Pasteur, Attenuated chicken cholera vaccine development
� 1883 Elie Metchnikoff Cellular theory of vaccination
� 1885 Louis Pasteur, Rabies vaccination development
� 1888 Pierre Roux & Alexandre Yersin, Bacterial toxins
� 1888 George Nuttall, Bactericidal action of blood
� 1891 Robert Koch, Delayed type hypersensitivity
� 1894 Richard Pfeiffer, Bacteriolysis
� 1895 Jules Bordet, Complement and antibody activity in bacteriolysis
� 1900 Paul Erlich, Antibody formation theory.
� 1901 Karl Landsteiner, A, B and O blood groupings
� 1901-8 Carl Jensen & Leo Loeb, Transplantable tumors.
� 1902 Paul Portier & Charles Richet, Anaphylaxis.
� 1903 Almroth Wright & Stewart Douglas, Opsonization reactions
� 1906 Clemens von Pirquet, coined the word allergy
� 1907 Svante Arrhenius, coined the term immunochemistry
� 1910 Emil von Dungern, & Ludwik Hirszfeld, Inheritance of ABO blood groups
� 1910 Peyton Rous, Viral immunology theory
� 1914 Clarence Little, Genetics theory of tumor transplantation
� 1915-20 Leonell Strong & Clarence Little, Inbred mouse strains
� 1917 Karl Landsteiner, Haptens
� 1921 Carl Prausnitz & Heinz Kustner, Cutaneous reactions
� 1924 L Aschoff, Reticuloendothelial system
� 1926 Lloyd Felton & GH Bailey, Isolation of pure antibody preparation
� 1934-8 John Marrack, Antigen-antibody binding hypothesis
� 1936 Peter Gorer, Identification of the H-2 antigen in mice
� 1940 Karl Landsteiner & Alexander Weiner, Identification of the Rh antigens
� 1941 Albert Coons, Immunofluorescence technique
� 1942 Jules Freund & Katherine McDermott, Adjuvants
� 1942 Karl Landsteiner & Merill Chase, Cellular transfer of sensitivity in guinea pigs (anaphylaxis)
� 1944 Peter Medawar, Immunological hypothesis of allograft rejection
� 1948 Astrid Fagraeus, Demonstration of antibody production in plasma B cells
� 1948 George Snell, Congenic mouse lines
� 1949 Macfarlane Burnet & Frank Fenner, Immunological tolerance hypothesis
� 1950 Richard Gershon and K Kondo, Discovery of suppressor T cells
� 1952 Ogden and Bruton, discovery of a gamma gobulinemia (antibody immunodeficiency)
� 1953 Morton Simonsen and WJ Dempster, Graft-versus-host reaction
� 1953 James Riley & Geoffrey West, Discovery of histamine in mast cells
� 1953 Rupert Billingham, Leslie Brent, Peter Medwar, & Milan Hasek, Immunological tolerance hypothesis
� 1955-1959 Niels Jerne, David Talmage, Macfarlane Burnet, Clonal selection theory
� 1957 Ernest Witebsky et al., Induction of autoimmunity in animals
� 1957 Alick Isaacs & Jean Lindeman, Discovery of interferon (cytokine)
� 1958-62 Jean Dausset et al., Human leukocyte antigens
� 1959-62 Rodney Porter et al., Discovery of antibody structure
� 1959 James Gowans, Lymphocyte circulation
� 1961-62 Jacques Miller et al., Discovery of thymus involvement in cellular immunity
� 1961-62 Noel Warner et al., Distinction of cellular and humoral immune responses
� 1963 Jacques Oudin et al., antibody idiotypes
� 1964-8 Anthony Davis et al., T and B cell cooperation in immune response
� 1965 Thomas Tomasi et al., Secretory immunoglobulin antibodies
� 1967 Kimishige Ishizaka et al., Identification of IgE as the reaginic antibody
� 1971 Donald Bailey, Recombinant inbred mouse strains
� 1974 Rolf Zinkernagel & Peter Doherty, MHC restriction
� 1975 Kohler and Milstein, Monoclonal antibodies used in genetic analysis
� 1984 Robert Good, Failed treatment of severe combined immunodeficiency (SCID, David the bubble boy) by bone marrow grafting.
� 1985 Tonegawa, Hood et al., Identification of immunoglobulin genes
� 1985-7 Leroy Hood et al., Identification of genes for the T cell receptor
� 1990 Yamamoto et al., Molecular differences between the genes for blood groups O and A and between those for A and B
� 1990 NIH team, Gene therapy for SCID using cultured T cells.
� 1993 NIH team, Treatment of SCID using genetically altered umbilical cord cells.
����������� 1985- onwards; identification of genes for immune cells, antibodies, cytokines and other immunological structures. Information provided by: http://www.keratin.com
Note- This material has been taken from various published books, journals and made available in Google browser system.� Students should be thankful to the authors and the published texts.