Eukaryotic System:
Co-Translation:
· Eukaryotic cells are bound by cell membranes as the outer envelope in animal cells, but in plants they have an additional protective structure in the form of cell wall. In both, the cell membrane or plasma membranes are living and dynamic, for they possess several hundred or more of proteins, which perform a variety of functions of importance. Membranes also contain a large number of integral proteins and they are also associated with extra cellular proteins at the outer surface and many at the cytosolic surface. In addition, they also contain receptors and transporters.
· Chemical composition of several kinds of membranes suggests that the lipid and protein ratio 50:50 to 25:75. They vary from one membrane of an organism to the other. Different membranes of different organisms contain different kinds of proteins and lipids; they are organized differently from one to another species. They are localized on either sides of the membrane and localized in inner core of the membrane. Proteins and phospholipids are specific to specific organism to organism. Specific enzymes called flippases, perform asymmetric distribution of many lipids and proteins, during their assembly. Membranes always grow from the existing membranes and often lose some part.
· The intracellular space often considered as microcosm of the cell, is highly compartmentalized with membranes, where each of the compartments has specific structures and unique functions. Many of the cellular lipids are synthesized at the cytosolic surface of endoplasmic reticulum and transported. Some organelles are involved in the synthesis of specific lipids, ex. HPl in Plastids. Millions, (yeast) perhaps more than 50x10^6 different types of proteins are found in organisms; they are synthesized in different numbers and kinds, as they are synthesized, they are transferred to different destinations and different target sites. This holds good for all types of membranes.
· Membranes are structural and functional components of a cell. And each of the said organelles or sites requires specific kind of proteins. Translational machinery has to cope with the synthesis of all these proteins and they have to be synthesized in different ratios and at different rates. Older ones and damaged ones have to be replaced. How this is achieved is yet to be completely unraveled. If I cannot explain I resort to say “God only Knows”, if GOD ever existed ?!!, This is a shear imagination of human mind, created the illusion of “GOD”. Scientist are no exception to this, take for example “God’s’ Particles” (Bosons).
In general, all those proteins, destined towards plasma membranes and secretion, are synthesized on ribosomes bound ER. Many intracellular organelle membrane proteins are also synthesized on ER, ex. Golgi body proteins, ER proteins, lysosomal proteins, endosomal and intracellular secretary vesicular membrane proteins, all are synthesized on Endoplasmic reticular membranes. Additional ER domains have been discovered and characterized.
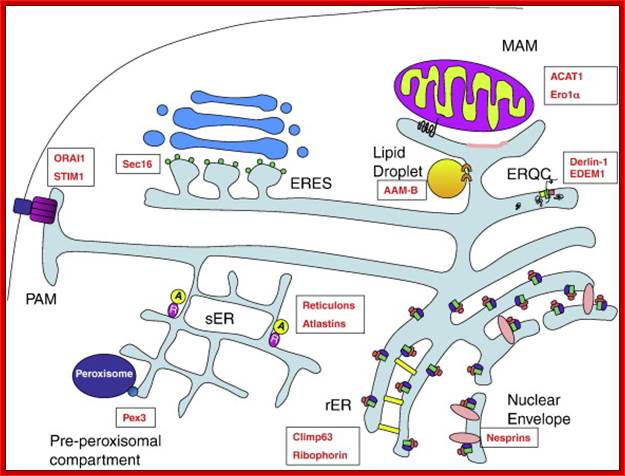
Fig; The ER subdomains, and selected markers: Rough Endoplasmic Reticulum (rER) sheets are characterized by ribosomes (pink ovals) which associate with translocon components (green rectangles) and ribophorins (purple ovals). Climp63 (yellow rectangle) is an important structural protein of the rER that also associates with microtubules. The ERQC (ER quality control compartment) is derived from the rER and also features translocon components (green rectangle) and associated proteins such as Derlin-1 (yellow square) and EDEM-1 (pink square) that facilitate the ubiquitination and retro translocation of misfolded proteins from the ER. Smooth Endoplasmic Reticulum (sER) tubule formation is in part mediated by the reticulons (purple circles) and atlastins (yellow circles). At the PAM (Plasma membrane-associated Membrane), STIM1 (purple rectangle) oligomerizes to form a pore which associates with the plasma membrane calcium channel ORAI1 (dark blue rectangle), mediating store operated calcium entry. The MAM (Mitochondria Associated Membrane) is a section of smooth ER that makes close contacts with mitochondria. ACAT1 (black squiggle) localizes to MAMs via a mitochondrial targeting sequence in its cytosolic tail, whereas other MAM proteins target to cholesterol-rich lipid domains within the MAM (pink membrane section). ER exit sites (ERES) at the transitional ER mark the point where COPII coated vesicles bud off from the ER en route to the ERGIC and Golgi compartments; ERES formation depends on Sec16 (small green circles), which associates with the ER membrane on the cytosolic face. Proteins destined for the peroxisomes sort into the pre-peroxisomal compartment, where they bud off into pre-peroxisomal vesicles in a Pex3 (small blue circle) -dependent manner. Lipid droplets are characterized by the presence of selected ER proteins including AAM-B. The nuclear envelope is equipped with ribosomes on the cytoplasmic face, where nesprins (pink ovals) are also found. http://www.tankonyvtar.hu
The mitochondria-associated endoplasmic reticulum membrane (MAM) is a specialized subdomain of the endoplasmic reticular (ER) membrane that regulates ER-mitochondria communications. The MAM is characterized by direct apposition to a mitochondrion, a unique lipid profile, and the expression of a unique set of proteins involved in Ca2+ signaling, phospholipid biosynthesis, protein folding, and membrane tethering. The association of the MAM with a mitochondrion is in part cytoskeleton independent and dynamically changed by an elevation of the cytosolic Ca2+ level. The mechanisms underlying the genesis of MAM are unclear but might involve COPI-dependent vesicular transport and soluble NSF attachment protein receptor. The MAM is recognized as a center for intermembrane transport of phospholipids and for direct Ca2+ transmission to mitochondria that activates the tricarboxylic acid cycle. However, MAM might be also involved in the interorganelle transport of cholesterol, ceramides, ATP, and proteins as well as in proteasomal protein degradation and lipid droplet formation. Recent studies have begun to unveil the importance of inter organelle communication in the innate immune response to virus infection and in the pathophysiology of neurodegenerative/neurodevelopmental disorders. Thus, drug discovery aimed at regulating ER-to-mitochondria communication may open a new avenue in treatments of human diseases; The mitochondria-associated endoplasmic reticulum membrane (MAM) is a specialized subdomain of the endoplasmic reticulum (ER) membrane that regulates ER-mitochondria communications. The MAM is characterized by direct apposition to a mitochondrion, a unique lipid profile, and the expression of a unique set of proteins involved in Ca(2+) signaling, phospholipid biosynthesis, protein folding, and membrane tethering. The association of the MAM with a mitochondrion is in part cytoskeleton independent and dynamically changed by an elevation of the cytosolic Ca2+ level. The mechanisms underlying the genesis of MAM are unclear but might involve COP-I dependent vesicular transport and soluble NSF attachment protein receptor. The MAM is recognized as a center for intermembrane transport of phospholipids and for direct Ca2+ transmission to mitochondria that activates the tricarboxylic acid cycle. However, MAM might be also involved in the inter-organelle transport of cholesterol, ceramides, ATP, and proteins, as well as in proteasomal protein degradation and lipid droplet formation. Recent studies have begun to unveil the importance of inter-organelle communication, in an innate immune response to virus infection and in the pathophysiology of neuro-degenerative/neuro-developmental disorders. Thus, drug discovery aimed at regulating ER-to-mitochondria communication may open a new avenue in treatments of human diseases.
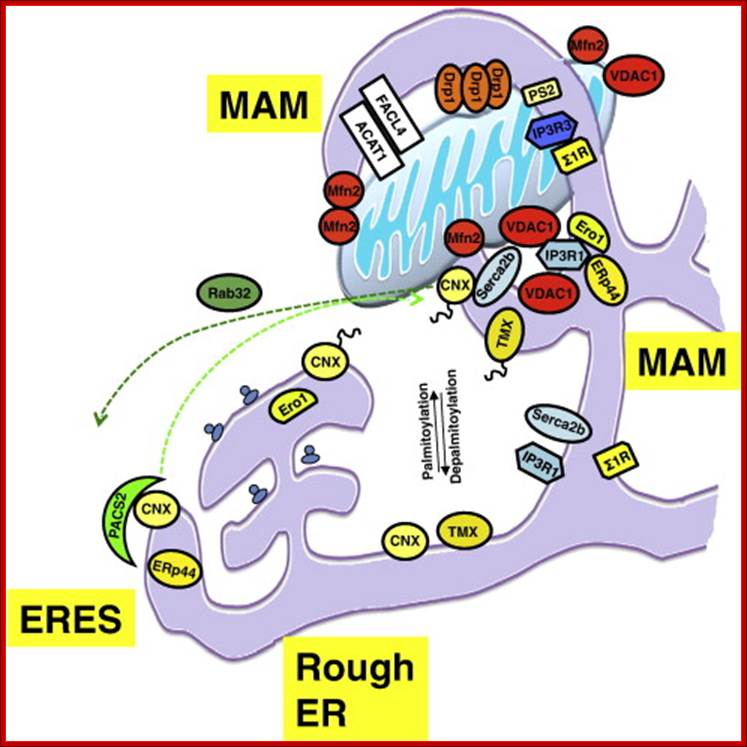
http://ars.els-cdn.com/content/image/1-s2.0-S0167488912001048-gr1.jpg; Diagram of mammalian MAM-enriched proteins, as summarized: Only bona fide mammalian MAM proteins are represented. The MAM is a hub for lipid metabolism (white; top portion of MAM), mitochondrial fission (orange; indicated by the Drp1 oligomers) and ER chaperones and oxidoreductases (yellow; bottom portion of MAM). Enrichment of MAM proteins to the vicinity of mitochondria is regulated by lipid modification (palmitoylation) and by cytosolic sorting proteins (green; PACS-2, Rab32). MAM tethering involves mitofusin-2, GRP75 (not shown) and VDAC1 (red). Calcium handling proteins such as SERCA2b, IP3R1 and IP3R3 are found on the MAM (blue), but do not appear specifically enriched here; Arun Raturi, Thomas Simmen
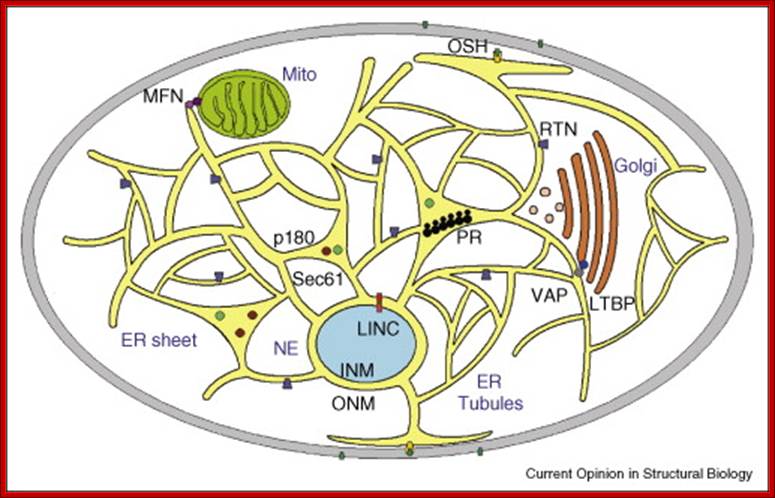
Resident ER proteins utilized by the cell to shape ER membrane and link it to other regions within the cytoplasm: Reticulons (blue shapes) and DP1/Yop1 proteins shape the tubular ER and p180 (brown circle), polyribosomes (black tracks), and components of the translocon complex (Sec61β- turquoise circle) shape the sheet-like ER, and the inner and outer membranes of the nuclear envelope are linked by the LINC complex (pink and red rectangles). The peripheral ER has numerous contact points with various membranes. The ER proteins form bridges with the mitochondria through MFN2/MFN1 dimers (pink and purple circles), with Golgi membranes through VAPs (gray circles) interacting with lipid transfer binding proteins (LTBP — bright blue circles), and the plasma membrane potentially through Osh proteins (gold shape, sterols are green circles), Amber R English*, Nesia Zurek etal.
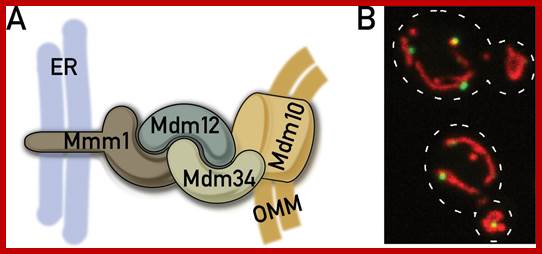
The ERMES complex and ER–mitochondria connections; Cellular organelles need to communicate in order to co-ordinate homoeostasis of the compartmentalized eukaryotic cell. Such communication involves the formation of membrane contact sites between adjacent organelles, allowing privileged exchange of metabolites and information. Using a synthetic protein designed to artificially tether the ER (endoplasmic reticulum) to mitochondria, we have discovered a yeast protein complex naturally involved in establishing and maintaining contact sites between these two organelles. This protein complex is physiologically involved in a plethora of mitochondrial processes, suggesting that ER–mitochondria connections play a central coordinating role in the regulation of mitochondrial biology. Recent biochemical characterization of this protein complex led to the discovery that GTPases of the Miro family are part of ER–mitochondria connections. The yeast Miro GTPase Gem1 localizes to ER–mitochondria interface and influences the size and distribution of mitochondria. Thus Miro GTPases may serve as regulators of the ER–mitochondria. Another proposed role for the ER–mitochondria connection is to promote interorganelle calcium (Ca2+) exchange. Connection, especially neurological connections. Agnès H. Michel, Benoît Kornmann; http://www.biochemsoctrans.org
These include the mitochondria-associated membrane (MAM), the ER quality control compartments (ERQC), where ER-associated degradation (ERAD) occurs, and the plasma membrane-associated membrane (PAM). Research has also shown that the biogenesis of peroxisomes and lipid droplets occurs on specialized membranes of the ER. Several studies have shown the existence of specific marker proteins found on all these domains and how they are targeted there --Emily M. Lynes, Thomas Simmen.
· Among all other membranes ER membrane is the extended and largest network of membranes can be called as an ‘organelle’. It has a continuous network of membranes involved in various functions. There are various domains such as SER and RER involved in lipid, carbohydrate metabolism and proteins synthesis respectively.
· ER is also often associated with Microtubules and intermediate filaments. Perhaps MTs help in ER extensions and movement.
· ER is also responsible for the formation peroxisomes via Pex3 and Pex19 via budding off from ER.
- But many embryonic protein syntheses are localized in the cell. Their mRNAs are in specific location of the embryo. The mRNAs are actually carried or ferried to their specific destinations within the cell through the binding of specific mRNA binding proteins to 3’UTR sequences, then they are transported along the microtubules or microfilaments as tracts, where motor proteins such as dyneins and or kinesin are employed for ATP dependent transport, ex. In Drosophila Nanos mRNAs are localized in the posterior region of the embryo and Bicoid mRNA are located in the anterior part of the embryo and Gurken in antero-dorsal region.
· Proteins for mitochondria, chloroplasts, peroxisomes and nuclei are synthesized in free-state, i.e., free from ER, and then they are then directed to their respective destinations using signal sequences and specific transporter proteins.
· There are other proteins of which some are soluble enzymes, some may act as factors, some are cytoskeletal elements and some may have mechanical functions; all of them are synthesized with in cytosol, but free from ER.
· The synthesis of these proteins is not without regulation, but the site or sites of synthesis of them are positioned within intracellular milieu.
Protein Synthesis on Endoplasmic reticulum:
- Initiation of translation takes place with ribosome binding to mRNAs in cytoplasm in free-state, i.e. free from ER.
- As the synthesis progresses the N- terminal segment of the polypeptide chain emerges out of the exit tunnel region of the large ribosome. The exit channel, 20Å diameter, can hold a chain of 35-40 amino acids long polypeptide.
- The amino acid sequence in the N-terminal region of the emerging polypeptide or specific sequences in the chain ultimately determine, whether or not the protein synthesis directed to ER or ER free.
Secretary or Membrane Protein Signal Sequences:
- Bovine growth hormone:
- H3N-MMAAPRTSLLLAFALLCLPWTQVVG-Human.pre-proinsulin:
- Human heavy chain of IgG: N-MEFGLSWLFLVALLKGVQCI. EVQ.
- Pre IgG light chain: N-MD.R…QIFGFLLLLFPGTRC- D.
- Rat amylase: N-MKFVLLLSLIGFCWA- QYD.
- Human interferon-MKYTSYILAFQLCIYLGSLG.
- Cyt. Oxidase: N-MLSLRQSIRFFKPATRTLCSSRYLL.
- Bovine pro albumin: MKWVTFISLLLLFSSAYS- RGV.
- Chick Lysozyme: MRSLLILVLCFLPLAALG- KVF.
- Pre-Lysozyme: N-MRSLLILVLCFLPLAALG- K
- ER resident protein: N-KDEL.
- Pre pro albumin: N-MK. FLLLLFISGSAFS- R.
- Pre-pro lactin: N- MNSQVSARKAGTLLLLMMSNL- L
- VSV protein: N-MKCLLYLAFLFIHVNC- K.
- Rat pro insulin: N-MALWMRFLPLLALLVLWEPKPA- F.
- Acetyl choline receptor g-subunit: N-MVLTLLLIICLALQVRS IQ
- Import into ER: N-MMSFVSLLLVGILFWATEAGELTKCE.
- Attached to membranes: Myristoyl-N-GSSKSKPK.
ER recognition sequences-Amino Acid Sequences of ER Signal Peptides in Three Eukaryotic Proteins, just few examples.
|
Protein |
Amino Acid Sequence* |
|
Preproalbumin |
Met-Lys-Trp-Val-Thr-Phe-Leu-Leu-Leu-Leu-Phe-Ile-Ser-Gly-Ser-Ala-Phe-Ser ↓ Arg . . . |
|
Pre-IgG light chain |
Met-Asp-Met-Arg-Ala-Pro-Ala-Gln-Ile-Phe-Gly-Phe-Leu-Leu-Leu-Leu-Phe-Pro-Gly- Thr-Arg-Cys ↓ Asp . . . |
|
Prelysozyme |
Met-Arg-Ser-Leu-Leu-Ile-Leu-Val-Leu-Cys-Phe-Leu-Pro-Leu-Ala-Ala-Leu-Gly ↓ Lys . . . |
Note: Downward arrow; the site at which signal sequences are cut by signal peptidases and remove signal sequence fragment.
Nuclear protein signal sequences:
- SV40 t antigen: PKKKRK V.
- Polyoma T antigen: PKKAR I ED.
- Polyoma middle T antigen: PVSRKRPRP.
- SV40 VP1: APTKRKGS.
- Nucleoplasmin: GQAKKKKLD (KR[PAATKKAGQA]KKKK)
Signals for Glyoxysomes-n-Peroxisomes:
· N-SKL.
Signals for mitochondria:
· NMLSLRQSIRFFKPATRTLCSSRYLT.
· Cyt. Oxidase IV: N-MLSLRQSIRFFKPATRTLCSSRYLL I.
· Cyt.C1: SNLSKRWAQRTLSKSFYSTATGAASKSGSG
KLTEKLVTAGVAAAGFTATLLTADSLTADA I
Signal Sequences:
|
Organelle |
Location of Signal
in the Protein |
Nature of Signal |
|
||
|
Endoplasmic Reticulum |
C-terminus |
KDEL or HDEL (in yeast) |
|
||
|
Mitochondrion |
N-terminal |
3-5 nonconsecutive Arg or Lys; often contains Ser & Thr; never has acidic amino acids; no consensus sequence. |
|
||
|
Chloroplast |
N-terminal |
Generally rich in Ser, Thr, and small hydrophobic amino acids; no acidic amino acids |
|
||
|
Nucleus |
Internal |
Single cluster of 5 basic amino acids (e.g. KKKRK in the SV40 T antigen), or 2 smaller clusters separated by 10 amino acids |
|
||
|
Lysosome |
Internal |
Covalent attachment of Mannose-6-phosphate |
|
||
|
Peroxisome |
C-terminus |
SKF tripeptide at C-terminus |
|
||
|
Human Signal Sequences from the SwissProt Database |
|
||||
|
|
|
||||
|
Entry # |
Description |
Sequence |
|||
|
P04216 |
THY-1 MEMBRANE GLYCOPROTEIN PRECURSOR (THY-1 ANTIGEN) |
MNLAISIALLLTVLQVSRG |
|||
|
P01266 |
THYROGLOBULIN PRECURSOR |
MALVLEIFTLLASICWVSA |
|||
|
P35590 |
TYROSINE-PROTEIN KINASE RECEPTOR TIE-1 PRECURSOR |
MVWRVPPFLLPILFLASHVGAAVD |
|||
|
P01033 |
METALLOPROTEINASE INHIBITOR 1 PRECURSOR |
MAPFEPLASGILLLLWLIAPSRA |
|||
|
Q16819 |
MEPRIN A ALPHA-SUBUNIT PRECURSOR (ENDOPEPTIDASE-2) |
MAWIRSTCILFFTLLFAHIAA |
|||
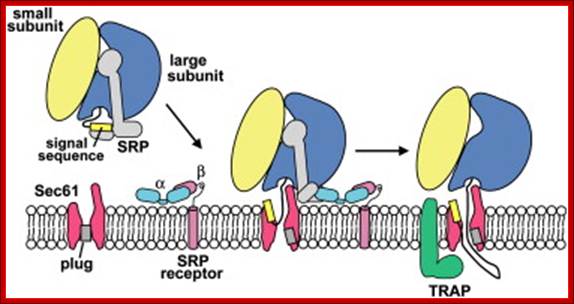
Binding of signal peptide sequences to SRP protein complex, the large ribosomal (80S) subunit is directed to ER and binds to ER resident proteins called ribophorins. However, the transport and positioning of the translating ribosome on to ER solely lies in characteristic features of SRP (Signal Recognition Protein) complex and ER resident docking or SRP-receptor proteins.
SRP complex:
- In a mammalian cell, the concentration of SRPs is one tenth of ribosome population. This complex acts at co-translation level.
- The SRP is 325 KD particles. It consists of 7sL RNA, transcribed by RNA-polymerase-III, of 300 to 305 ntds long and nearly 80 percent of its length is in double stranded secondary structural form with 6-7 bulges and 7-8 stems with 5’ and 3’ ends facing each other at the basal region.
- This highly structured RNA can be cleaved in the center by micrococcal restriction endonuclease called Alu-1, which produces one segment called Alu domain and the other S-domain. The Alu domain has no functional features but the S-domain has functional features.
- This 7sL RNA, secondary structured, is associated with six polypeptides bound to different domains of the dsRNA in sequence specific manner.
- From left side of the structured 7SL RNA, polypeptides bound are p9/p14, in the middle region one finds p68/p72 kDa subunits and p54 and p19 at terminal (numerical numbers denotes molecular eight).
- The p9/p14 is located near the base of 7sLRNA; p68/p72 is bound to the neck region of the dsRNA. The protein p19 is located at the large branched lobes of the RNA and p54 is associated with one of the branches where p19 is also bound.
The p54 protein has cleft like structure with several sites at which hydrophobic chains of CH2CH2S (CH3) are covalently linked as clusters, which actually provides strong hydrophobic charges. The p54 also contains a GTP binding site. It is the p54 region with its hydrophobic amino acids recognizes hydrophobic signal sequences in the exciting polypeptide chain as it emerges out of the large ribosomal exit tunnel; binding can be at N-terminal or in the middle or C-terminal, depends upon the position of hydrophobic signal sequences in the polypeptide chain.
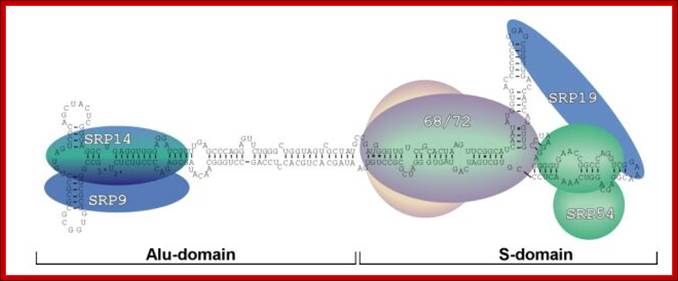
The SRP complex consists of 7s Sc RNA divided into two domains one Alu and the other S domain and several RNA binding proteins six in numbers. Each of the proteins recognizes a specific sequence of Sc RNA in secondary structure and bind. These proteins and the ScRNA are responsible for interacting with the emerging N-terminal region of the proteins through the ribosomes; if the N-terminal sequences have signal sequences then they bind and transfer on to ER surface.
This diagram shows the sequence dependent secondary structure with 5’ end starting with 5’G and end at UAG3’. The Alu domain is on the left side with T-shaped arm and the S-domain is on the right side, which is clearly distinguished in the above diagram. http://designmatrix.wordpress.com/
- When SRP complex recognizes and binds to terminal or subterminal hydrophobic region (signal sequence) of the nascent polypeptide chain that emerges out of exit tunnel, P9 and P14 dimers curve on ribosomal surface and arrest translation temporarily.
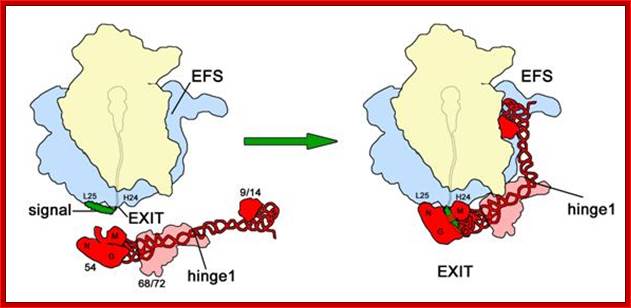
Upon signal sequence binding by SRP54, a kinked conformation of SRP is stabilized involving possibly SRP68/72 and a rotation around hinge 1. As a result, SRP interacts with the ribosome, stretching from the peptide exit (S-domain) to the elongation factor binding site in the intersubunit space (Alu-domain), where it causes elongation arrest by competition with elongation factors. Colour code as in figure 2; signal, signal sequence (green); EXIT, peptide tunnel exit; EFS, elongation factor binding site. Mario Halic; http://edoc.hu-berlin.de/http://edoc.hu-berlin.de/
- While the protein chain is elongating with N terminal region lacking in any signal sequences protein continues to elongate, then if some sequences contain hydrophobic sequences emerge out of ribosomal exit tunnel; then the SRP’ proteins’ p54 bind. At this point triggering factor activates out-coming protein.
- The p68 and p72 are bound to the neck region of 7sL RNA. These proteins recognize ER bound receptors or docking proteins.
- The p54 with strong hydrophobic charges interact and bind only to the N-terminal hydrophobic sequences or any such internal sequences called signal sequences that come out of the ribosomal channels. If the projected N-terminal sequence or in any positions of the protein chain does not contain hydrophobic signal sequences, the SRP won’t recognize them. Most of such proteins are destined to be either in free state in the cytosol or destined to organelles or depending upon the position of hydrophobic sequences.
- Most of the signal sequence regions contain amino acids such as Leu, Val, Ile, Ala; they actually form a core sequence. The length of signal sequence can vary from 20 to 30 or more.
- The hydrophobic amino acid signal sequences are not conserved; it varies from one protein species to the other, but the overall sequences should be hydrophobic in nature.
- The endoplasmic SRP-receptor protein or docking protein contains SR α (69 KD) and SR β (30KD) subunits.

SRP is universally conserved and comprises two functional
domains: The Alu and
the S domain. The S domain confers specificity to the process by
recognizing the signal sequence of ER-targeted proteins and by targeting the
nascent chain to the translocon via its interaction with the SRP receptor (SR
or docking protein) in the ER membrane. GTP binding and hydrolysis control
these critical steps. The Alu domain
interacts with the ribosome to slow down nascent chain elongation (elongation
arrest activity). It is responsible for the tight coupling of translation and
translocation. In the in vitro translation/translocation
system, this function increases the translocation efficiency, presumably by
lengthening the time window for the successful targeting of the nascent chain.
To study SRP functions in vitro, we reconstitute
SRP from recombinant wild type and mutated proteins and synthetic RNA and
examine the activities of the reconstituted particles in the in a cell-free
translation/translocation assay. In such experiments, we have identified a
tertiary structure in the Alu domain
of SRP which is important for SRP RNA folding and therefore for the assembly of
functional SRP. Currently, we are working on the characterization of a
C-terminal motif in SRP14, which we found to be essential for elongation arrest
activity of SRP and on other RNA motifs required for SRP functions.; http://cms2.unige.ch/
The N-terminal region of 69KD protein of Translocon is anchored in the membrane and the 30kd C-part is projected out into cytoplasmic face. The 30KD protein has GTP binding site and GTPase activity. The C-terminal face of the large protein interacts with 7sL RNA of the SRP 54p complex with its receptor domain. The 54-kD subunit of the signal recognition particle (SRP54) binds to signal sequences of nascent secretory and transmembrane proteins. SRP54 consists of two separable domains; a 33-kDa amino-terminal domain that contains a GTP-binding site (SRP54G) and a 22-kDa carboxy-terminal domain (SRP54M) contain binding sites for both the signal sequence and SRP RNA.
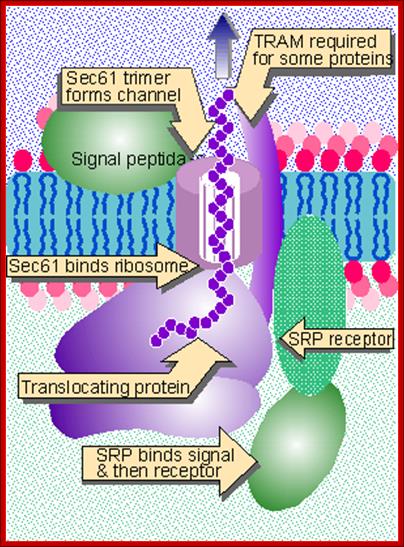
- The endothelial network membranes (ER network) also contain protein translocator protein complex associated with TRAM-Proteins (TRanslocation Associated Membrane Proteins). The translocator complex consists of Sec proteins like Sec61α, Sec61β and Sec61γ. The sec 61α has ten-membrane-pass domain. The trimers form a channel in the membrane with a 5-6nm high, diameter of 8.5nm and 2 nm hallow of the channel; the complex is called Translocon. The TRAM proteins that associate to form translocon assist the apparatus in transferring the protein into the ER lumen. The translocon is involved in both inward translocation but also outward translocation, but the mechanisms are different.
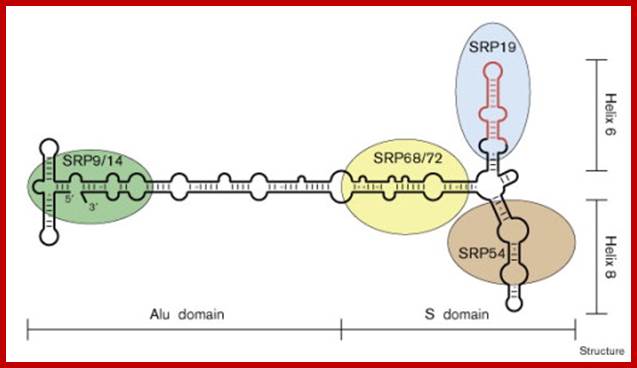
Schematic drawing of the human signal recognition particle showing the structural organization of the ribonucleoprotein complex. The RNA fragment of 29 nucleotides comprising the major binding site of protein SRP19 and including the closing tetraloop is highlighted in red. SRP proteins at their approximate binding sites are indicated in different colors. http://www.sciencedirect.com/
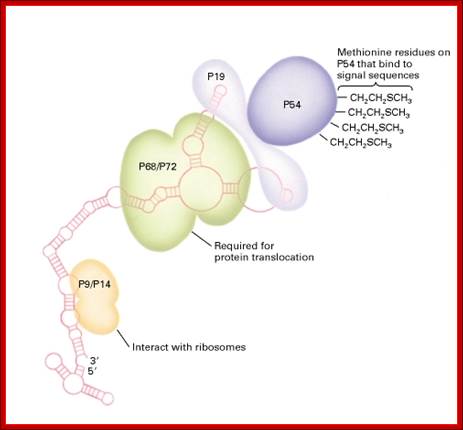
Structure of the signal-recognition particle (SRP):
SRP comprises one 300-nucleotide RNA and six proteins designated P9, P14, P19, P54, P68, and P72. (The numeral indicates the molecular weight × 10−3). All proteins except P54 bind to the RNA; the precise binding site of P9 and P14 is not known. Different functions have been assigned to the different polypeptides as indicated. In addition to binding the hydrophobic side chains of signal peptide sequences, P54 functions with α subunit of the SRP receptor to hydrolyze GTP. [See K. Strub et al.],
Protein Translocation into ER via ER located Translocon complex and SRP-translating ribosome complex:
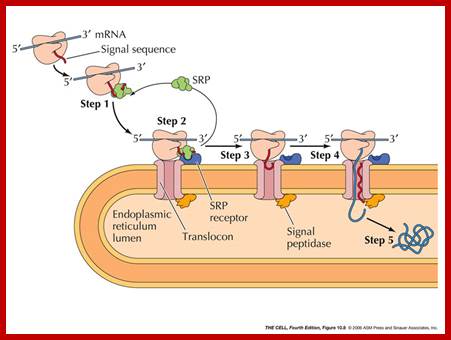
http://oregonstate.edu
Translocation into the ER: Post- and cotranslational translocation requires four steps. 1) Targeting to the ER membrane, which can be SRP-dependent or independent. Post-translationally translocated preproteins require molecular chaperones to maintain their solubility. 2) Insertion into the Sec61 translocon. 3) Energy-dependent import through the translocon. 4) Protein folding in the ER lumen, which may be chaperone-dependent, NCBI.
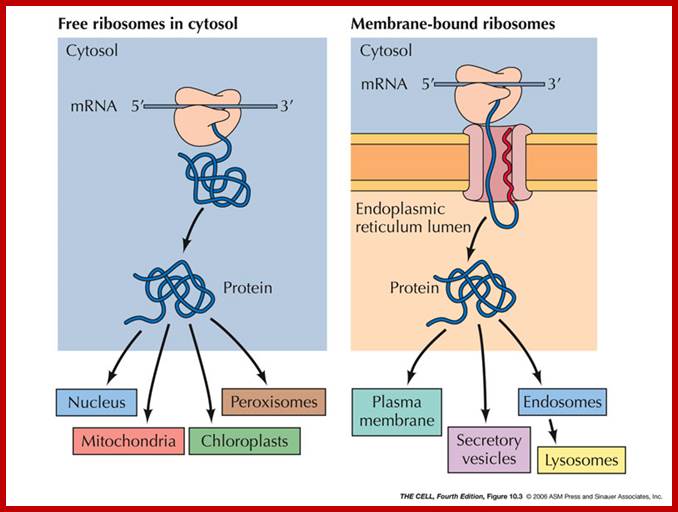
ER Associated Proteins and Rough ER:Some proteins are targeted for the lumen of the ER or to be embedded in its membrane. (Their ultimate fate may be different--they may be transported elsewhere later.) Sending a protein into the ER is the process of translocating it across (or into) the ER membrane. All protein synthesis begins on ribosomes in the cytosol which are unattached to the ER (free ribosomes). Proteins that are destined to remain in the cytosol complete their synthesis on free ribosomes and are therefore released into the cytosol. http://faculty.samford.edu
Secretory proteins enter the ER after or concomitant with their synthesis on cytoplasmic ribosomes in a process known as translocation. In either case, nascent secretory proteins must be targeted to the translocation machinery at the ER membrane and must traverse the lipid bilayer of the ER through the translocation channel. Molecular chaperones in the cytosol and ER lumen assist translocation and facilitate protein folding and assembly in the lumen. Proteins that achieve their native conformation exit the ER and continue through the secretory pathway. Incompletely folded or unassembled proteins are recognized by a constitutively active quality control pathway in the ER that identifies aberrant proteins and targets them for destruction in the cytosol by the proteasome. This process is known as ER associated degradation (ERAD). Sheara W. Fewell and Jeffrey L. Brodsky:
During cotranslational protein translocation, the ribosome associates with a membrane channel, formed by the Sec61 complex, and recruits the translocon-associated protein complex (TRAP).The Initiation of Protein Translocation at the ER in Higher Eukaryotes, a three-step model is shown for the docking of a ribosome-nascent chain-SRP complex to the ER membrane SRP receptor and subsequent formation of an active ribosome-channel complex, Jean-François Ménétret1, Ramanujan S. Hegde et al.
Binding of the SRP to receptor leads to positioning of the ribosomal tunnel-exit to sit on the translocon. The SRP receptor called SR anchored at the side of Sec 61 translocon complex in ER, consists of 69kDa (70kDa) alpha and 30kDa (25kDa) beta subunits. The SR alpha is a peripheral unit and SR 69 is membrane anchored protein. SR alpha binds to SRP54 subunit of the complex. Both SRP 54 and SR beta contain GTP binding sites (GTPase domain). If the binding is proper such that the ribosomal exit tunnel fits into the opening of ER translocon so that no other molecules can enter. When the placement of the protein synthesizing ribosome is placed properly, GTP bound to p54 SRP and GTP bound to alpha receptor hydrolyze to their respective GTPs to GDP + pi. This makes the ribosome tunnel sit tightly on the translocon complex.
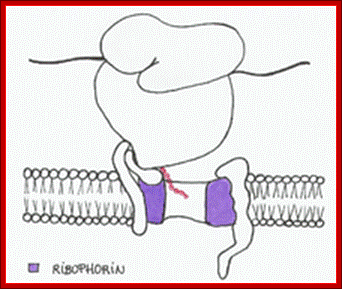
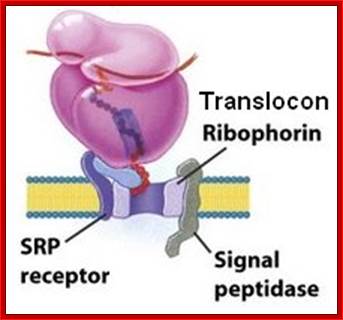
Ribophorin is transmembrane glycoprotein (subunit of oligosaccharide transferase) on RER, but not on SER. There are two types of Ribophorins RibI (20a.a) and RibII (22a.a), both are glycoproteins. Both of them luminally disposed They interact with ribosomes and aid in ribosome binding and protein translocation; (NCBI).
Present view of protein translocation across the ER membrane. The signal peptide, emerging from the ribosome, binds to the signal-recognition particle (SRP). The SRP-ribosome complex then docks to the SRP-receptor and channel ("translocon"). SRP dissociates from the receptor and the nascent polypeptide chain is translocated through the channel into the ER lumen. The signal peptide is finally cleaved and the protein is secreted out of the cell Copyright @ Nobel Media AB 2013
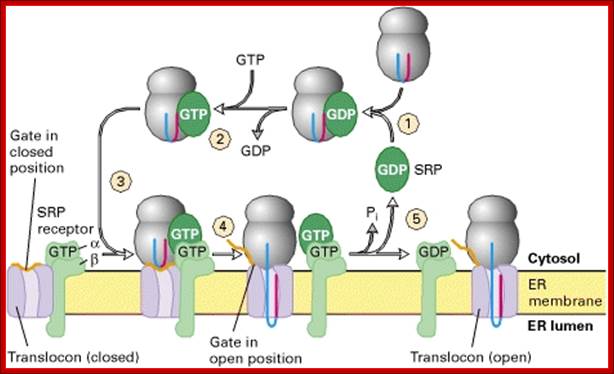
Cycles of GDP-GTP exchange and GTP hydrolysis that drive insertion of nascent secretory proteins into the translocon;
Both the P54 subunit of SRP and the α subunit of the SRP receptor bind and hydrolyze GTP. In step 1, SRP containing a bound GDP binds to the complex of a signal sequence and ribosome, triggering release of GDP by SRP and binding of GTP (step 2). The complex of SRP, ribosome, and signal sequence binds to the form of the SRP receptor in which the α subunit has a bound GTP (step 3). Hydrolysis of GTP by SRP and the SRP receptor then powers transfer of the nascent polypeptide, with its signal sequence, to the translocon and opening of the translocon “gate,” as well as release of SRP and dissociation of the SRP receptor from the translocon. An unknown protein then promotes release of GDP from the α subunit of the SRP receptor and binding of GTP (not depicted). [Adapted from T. Powers and P. Walter, 1996, Nature381:191, and G. Bacher et al., 1996, Nature381:248. For another model of how GTP hydrolysis powers assembly of the nascent chain–translocon complex see J. S. Millman and D. Andrews, 1997, Cell89:673, and P. Rapiejko and R. Gilmore, 1997, Cell89:703.]
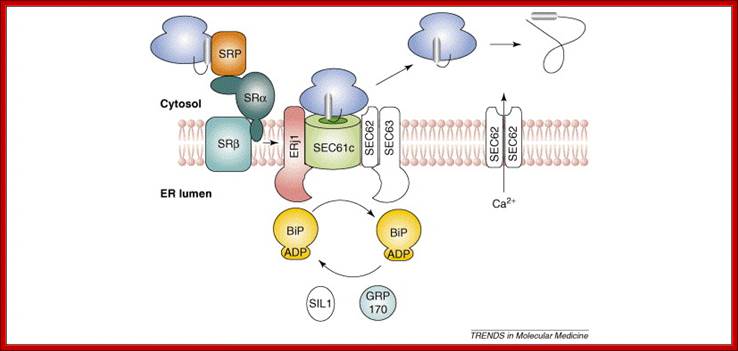
Co-translational transport of precursor polypeptides across the mammalian ER membrane. Transport of precursor polypeptides with N-terminal signal peptides is depicted in its three stages (from left to right): specific membrane association, membrane insertion and completion of translocation. The first stage or targeting reaction involves SRP and its receptor (SR). The second and third stages involve the protein translocase with its central subunit, the SEC61 complex and the additional subunits that are present in the membrane (SEC63 and ERj1), and provide a link to the ATPase cycle of the lumenal BiP via their J-domain (J). Furthermore, two alternatively acting nucleotide exchange factors (NEF) enable BiP to complete its functional cycle (SIL1 and GRP170). Typically, signal peptides are removed from the precursor polypeptides during their transit by signal peptidase (Spase). Note that the HSP40s and nucleotide exchange factors are not present in the right part of the figure to simplify the drawing.. Hypothetical model for cotranslational and SRP & SR dependent protein transport into the mammalian ER; Note that cleavable signal peptides within nascent precursor proteins insert into the Sec61 complex in a loop like fashion that orients the amino terminus to the cytosol and the carboxy-terminus plus the SPC cleavage site to the ER lumen: Richard Zimmermanna,,Susanne Eyrischb, Mazen Ahmadb, Volkhard Helmsb
Translocon:
Sec61 is the major component of the translocon. In detergent (which provides a hydrophobic milieu that mimics the effect of a surrounding membrane), Sec61 forms cylindrical oligomers with a diameter of ~85 and a central pore of ~20. Each oligomer consists of 3-4 heterotrimers (Hanein et al., 1996). The translocon forms an aqueous, ion conducting channel. The nascent chain that is translocated through ER translocon is in aqueous environment. The translocon is a dynamic structure with expanding inner diameter 9-15A to 40-60A in active state where ribosome is bound to translocon. The ribosomal tunnel is approximately 20A in diameter. BiP performs translocon gating cycle in ATP dependent mode. Bip is now known to bind and seals the inner end of translocon tightly. Only when 30-50 aa long N terminal end of the nascent polypeptide chain enters, BiP allows the protein to enter.
Electron
micrograph of
the protein translocating channel (the "translocon").
The translocon consists of SRP, SRP receptor, Sec61, TRAM, and signal peptidase, Lewin .B GenesVII.
ER bound translocon assist in transferring polypeptide chains, but also permeable to small ions. Protein translocons of the mammalian endoplasmic reticulum are composed of numerous functional components whose organization during different stages of the transport cycle in vivo remains poorly understood. We have developed generally applicable methods based on fluorescence resonance energy transfer (FRET) to probe the relative proximities of endogenously expressed translocon components in cells. Examination of substrate-engaged translocons revealed oligomeric assemblies of the Sec61 complex that were associated to varying degrees with other essential components including the signal recognition particle receptor TRAM and the TRAP complex. Remarkably, these components not only remained assembled but also had a similar, yet distinguishable, organization both during and after nascent chain translocation. The persistence of preassembled and complete translocons between successive rounds of transport may facilitate highly efficient translocation in vivo despite temporal constraints imposed by ongoing translation and a crowded cellular environment, Erik L. Snapp,et al.
A proposed model for chaperone-assisted folding of apoB to form VLDL- an example: Molecular chaperones interact with apoB as it emerges through the translocon (shown in red) into the ER lumen. Following elongation, domains which are presumably enriched in amphipathic β-sheets interact with the membrane cotranslationally, where initiation of MTP-mediated lipidation leads to the formation of a small lipid core (shown in yellow) (step I). Ongoing translation-coupled lipidation leads to elongation, enlargement of the core, and recruitment of additional molecular chaperones (step II). Recruitment of additional neutral lipid molecules leads to completion of translation followed by dissociation of the ribosome (step III). At this stage enough lipids had been recruited and the lipidated intermediate begins to detach from the ER membrane. It appears, however, that complete detachment of this intermediate is dependent on additional recruitment of chaperone molecules. These include GRP94, ERp72, and CyPB (cyclophilin B), but not CRT (calreticulin. These chaperones appear to facilitate the release presumably by replacing the hydrophobic interactions with the membrane and stabilizing the primordial intermediate following its release into the lumen (step IV). This intermediate fuses with a lipid droplet rich in TAG to form much larger particles (step V). This incompletely understood process occurs either in the ER or in a post-ER compartment. Prior to ER exit, a fraction of the bound chaperones dissociates (approximately 50–80% of CRT, GRP94, ERp72, and over 90% of BiP and CyPB), and the lipoprotein particles are transported to the Golgi (step VI). ER-resident chaperones remain associated with apoB-containing lipoproteins in the Golgi while additional modifications take place (step VII). Ultimately, all chaperones dissociate presumably before packaging into secretory vesicles (step VIII) and nascent VLDL is secreted, Haya Herscovitz, Ph.D.
Mechanism of Co-translation:
- In this process protein is translocated while the protein is being synthesized, hence the name co-translation and co-translocation.
- As the N-terminal part of the nascent polypeptide threads through the exit region of the ribosomal tunnel, if the sequences have core hydrophobic amino acids, the 54KD region of SRP complex, because of its hydrophobic side chains, interact and associate and bind to signal sequences.
- In this reaction, the 7sL RNA of SRP complex and rRNA of large subunit interact and stabilize the binding.
- Binding of p54 (SRPs are same in all members of eukaryotes) to N-terminal signal sequences of nascent protein N-terminal ends leads to the association of 9KD and 14KD (Alu domain) of SRP complex with ribosome and arrest chain elongation for the time being. If the N-terminal region does not contain the said signal sequences, the SRPs don’t bind and chain elongation continues and the protein is released into cytoplasm.
- Endoplasmic reticulum on its surface contains SRP-receptor proteins or docking proteins. The docking protein, as mentioned earlier, consists of two domains, one large subunit (18 membrane pass domain) and the other 30KD. The large subunit is anchored in the ER membrane; bulk of it is towards cytosolic surface. This part has domain that recognizes 7sL RNA-RNP. The SRP-ribosome complex recognizes the docking proteins and binds to 72 KD region of the receptor. This binding is assisted by ribophorins found in ER.
- These docking proteins are positioned next to ion channels made up of Sec 61 trimers assisted by proteins (it has 10 membrane pass motif), called TRAMP (Transport Associated Membrane Protein). The channel protein is also associated with sec62 and 63.
- One of the docking proteins, p30 has GTP binding site and it acts as GTPase.
- The channel is made up of at least six proteins of which some act as channels. A protein found at the luminal side of the translocon acts as signal peptidase.
- The docking of SRP-ribosome translating complex on to the docking proteins is so designed that the N-terminal hydrophobic part of the polypeptide is virtually aligned with the ion channel and they are threaded into it. If not ribosome gets free of docking complex and the protein synthesis continues and the protein is released free into cytoplasm for they have different destination and different mode of transportation to their respective destination.
- When the ribosome binds to the docking protein it positions in such a way, the ‘exit’ site of the large ribosomal subunit is in line with the channel in the ER translocon.
- When ribosome positions on to the translocon, it is totally blocked and prevent any entry or exit of any substances through it. The binding of the SRP-ribosome complex to docking proteins leads to GTP hydrolysis not only the GTP of docking protein but also of the GTP-of p54 complex. This releases the SRP from the docking proteins and SRP frees itself from the ribosome.
- This event also reactivates translation and polypeptide chain again starts elongation.
- The channel opens only when ribosomes are completely docked, otherwise they remain closed. The ion channel has a hydrophobic inner surface.
- If the hydrophobic sequences are located little away from the N-terminal, this is captured by the SRP complex and threaded into the translocon. In this process N-terminal part remains at the cytosolic side. The rest of the protein is threaded into ER lumen. Such proteins are membrane anchored or secretory proteins.
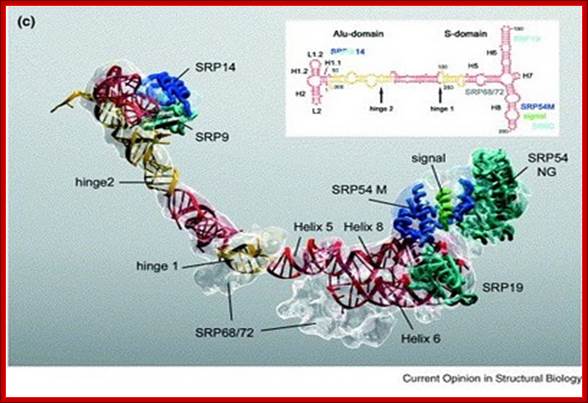
Molecular Model of SRP; SRP particle- associated with 7sLRNA; http://edoc.hu-berlin.de/
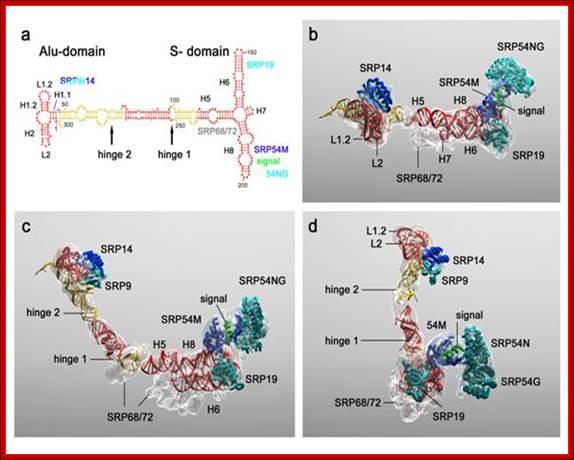
Molecular model of SRP; a, Secondary structure of the SRP RNA with protein binding sites and hinges indicated. H1-H8 denote the RNA helices of SRP, following the nomenclature of Weichenrieder et al]. In the case of the Alu-domain. Cyan, blue and grey, SRP proteins; red and yellow, 7S RNA; green, signal sequence. b, Molecular model of SRP with density transparent and colour coding as in a. Top view showing SRP as seen from the ribosome. c, As b but rotated upwards. d, As c but rotated left. http://edoc.hu-berlin.de/
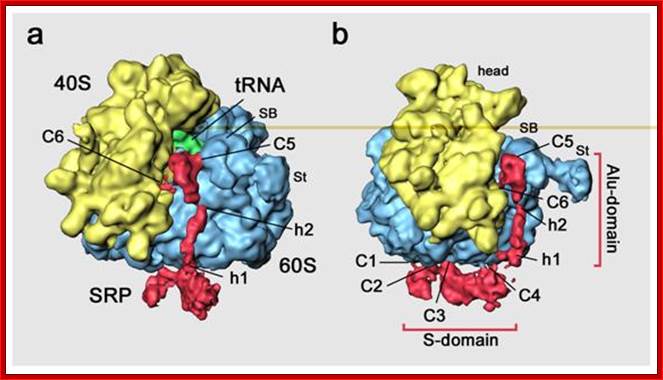
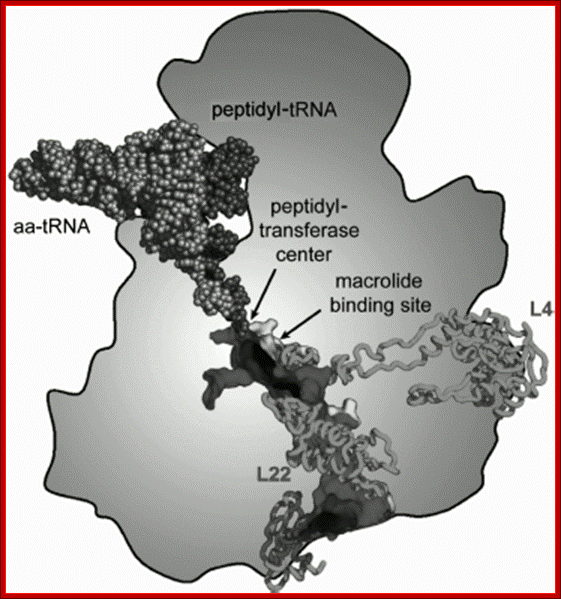
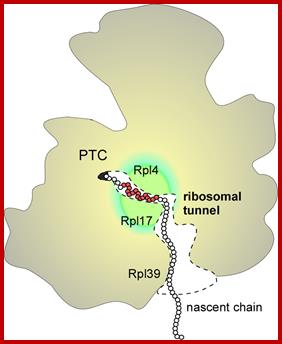
Fig: Scheme of arrangement of the ribosomal tunnel in the ribosome 50S subunit (cross-section along long axis of the RT). The macrolide binding site and proteins involved in the RT wall formation are shown (the upper part of the RT is shown in more detail in Fig. above A. A. Bogdanov*, N. V. Sumbatyan, A. V. Shish kina, V. V. Karpenko, and G. A. Korshunova-Adapted with permission from 86. Mankins, A. S. (2008) Curr. Opin. Microbiol., 11, 414-421.
The length of the ribosomal tunnel is directly related to the size of the large ribosomal subunit and it’s rRNA: in bacterial ribosomes it is approximately 90 Å, in eukaryotic ribosomes about 100 Å, and in mitochondrial ribosomes it is about 60 Å. At the same time, the RT (Ribose Tunnel) diameter in all ribosomes, independently of their source, is the same. It is approximately 15 Å in the upper (adjacent to PTC) third of the RT, in the middle part the tunnel narrows down 10 Å diameter, and then the tunnel expands again and a funnel-shaped structure with maximal diameter of approximately 25 Å is formed at its outlet. Specific amino acid residues are lined along the exit tunnel to facilitate smooth movement of the polypeptide chain. In addition, the tunnel is filled with water and other solutes. Whether the ribosomes are seeped with cytoplasmic solvent or not, is not known. The answer is that it is through signal sequences in proteins and SRP proteins and the translocon facilitate translocation through sec channels in ER (described above). The ribosome is often called as ‘Translatosome”.
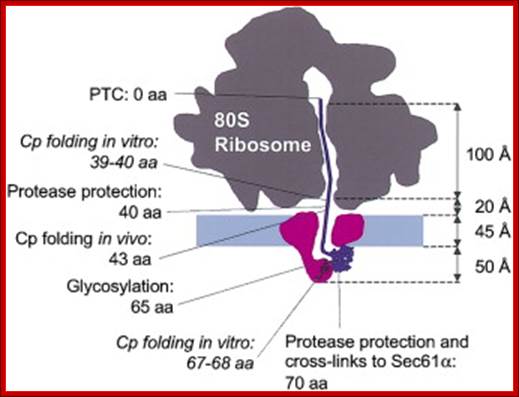
Protein Folding during Cotranslational Translocation in the Endoplasmic Reticulum;
;To test how far into the protein-conducting channel of the translocon complex a nascent polypeptide domain must move before it can fold, we analyzed the folding of in vitro translated products of truncated mRNAs encoding the Semliki Forest virus capsid protease domain (Cp) during translocation into microsomes. Cp folded when the C-terminal linker connecting it to the peptidyltransferase center was 64 amino acids or longer. This means that to fold, Cp must exit the translocon channel. With an uncleaved signal sequence, about one out of four of the Cp domains could undergo folding with a C-terminal linker of only 38–66 amino acids. This suggested that the constraint imposed on folding by the translocon complex may be less stringent for signal-anchored membrane proteins. Michael Kowarik, ;Stephanie Küng et al; http://www.cell.com
www.keywordsuggests.com
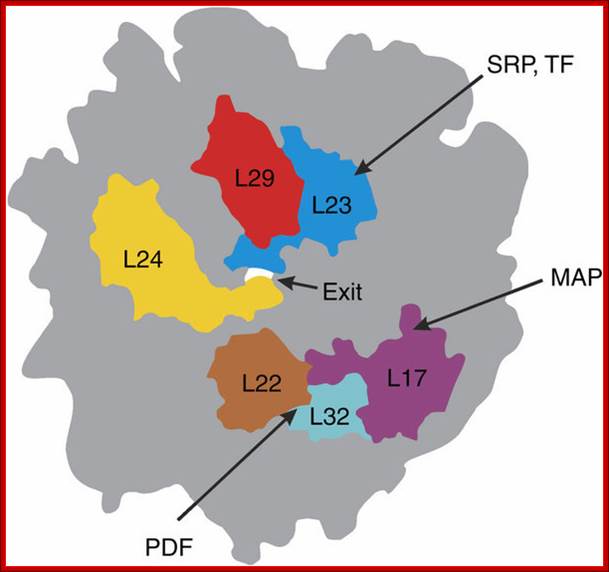
Nascent proteins emerging from translating ribosomes in bacteria are screened by a number of ribosome-associated protein biogenesis factors, among them the chaperone trigger factor (TF), the signal recognition particle (SRP) that targets ribosomes synthesizing membrane proteins to the membrane and the modifying enzymes, peptide deformylase (PDF) and methionine aminopeptidase (MAP). Here, we examine the interplay between these factors both kinetically and at equilibrium. TF rapidly scans the ribosomes until it is stabilized on ribosomes presenting TF-specific nascent chains. SRP binding to those complexes is strongly impaired. Thus, TF in effect prevents SRP binding to the majority of ribosomes, except those presenting SRP-specific signal sequences, explaining how the small amount of SRP in the cell can be effective in membrane targeting. PDF and MAP do not interfere with TF or SRP binding to translating ribosomes, indicating that nascent-chain processing can take place before or in parallel with TF or SRP binding.
Interplay between Trigger factor and other factor on ribosome. Thomas Bornemann, Wolf Holtkamp ,& Wolfgang Wintermeyer; ;http://www.nature.com/
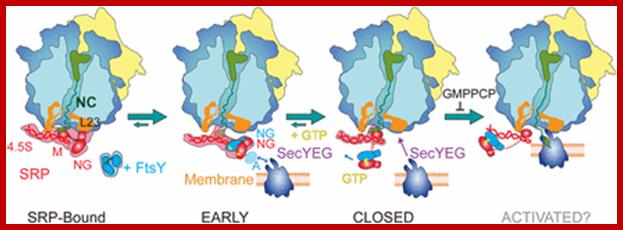
Model of cotranslational targeting in E. coli. In the RNC–SRP complex, SRP is prepositioned to bind FtsY. FtsY binding leads to an early complex inactive in GTP hydrolysis. Rearrangement of the NG domains in the closed complex requires GTP and leads to detachment of the NG domains from the RNA tetraloop. SecYEG binding to L23 is suggested to lead to docking of the NG domains to the distal end of the RNA in the activated state and RNC handover to SecYEG. GTP hydrolysis results in SRP–FtsY disassembly.
;http://www.pnas.org
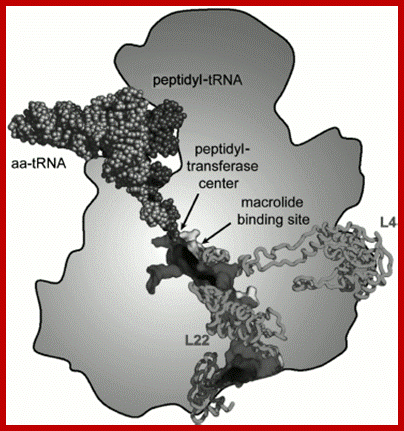
Scheme of arrangement of the ribosomal tunnel in the ribosome 50S subunit (cross-section along long axis of the RT). The macrolide binding site and proteins involved in the RT wall formation are shown (the upper part of the RT is shown in more detail in Fig.). http://www.protein.bio.msu.ru
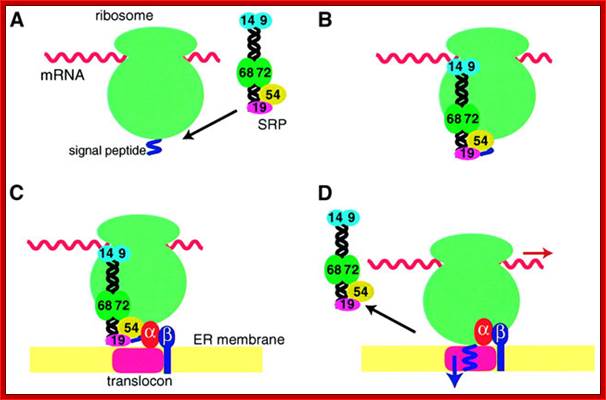
Figure is a simple representation of different protein subunits of SRP complex binding to different structures as protein chain is exiting out of the ribosomal tunnel. http://emboj.embopress.org/
Polypeptides binding to the SRP momentarily halts translations. The SRP excorts the ribosomes to the ER by binding to the SRP; http://www.studyblue.com/
Signal sequence recognition and co-translational targeting by SRP; Structures of protein targeting complexes; http://edoc.hu-berlin.de/
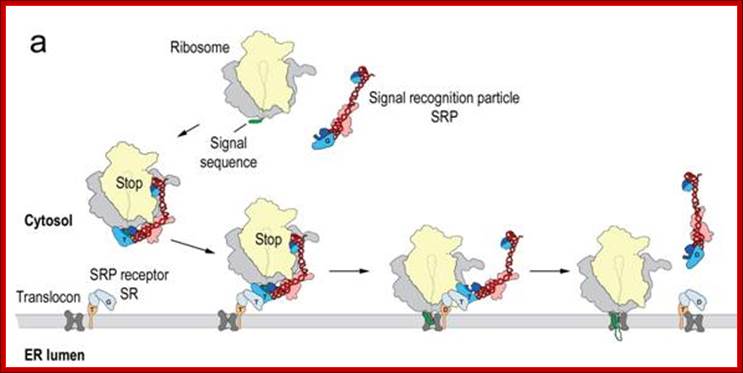
(a) Schematic overview of cotranslational targeting of proteins destined for secretion or membrane insertion. SRP interacts with the signal sequence as soon as it emerges from the ribosomal polypeptide exit tunnel (step I). In eukaryotes peptide elongation pauses upon SRP / ribosome nascent chain (RNC) complex formation and the RNC complex is targeted to the ER membrane by the interaction with the SR (step II). GTP binding to SRP and SR has been shown to be a prerequisite for SRP/SR complex formation. The RNC is then transferred to the protein-conducting channel in the membrane (the translocon) (step III) and triggered by GTP hydrolysis in SRP and SR the SRP/SR complex dissociates (step IV).
(b) Schematic overview of the mammalian SRP bound to the signal sequence carrying 80S ribosome (RNC) based on a cryo-EM structure. The SRP core as part of the S-domain is positioned near the tunnel exit of the large ribosomal subunit. The 40S and 60S ribosomal subunits are yellow and grey, respectively. The SRP RNA is shown in red and the SRP proteins are labeled as follows: SRP54NG (turquoise), SRP54M (dark blue), signal sequence (green), SRP19 and SRP68/72 (pink), SRP9/14 (turquoise/dark blue).
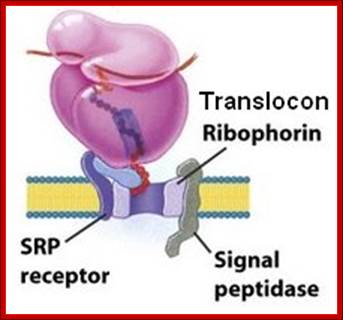
The three membrane components are the SRP receptor, the translocon (formerly known as ribophorin), and the signal peptidase. There are two subunits in the SRP receptor, α (docking protein) and β. The human genome contains a single gene for SRP receptor α subunit called SSPR (SSPRα). The genome has two separate genes for the β subunit called SSRB and SSR2.
http://sandwalk.blogspot.in/
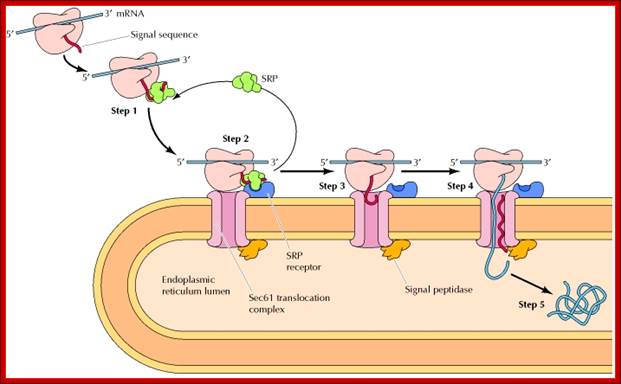
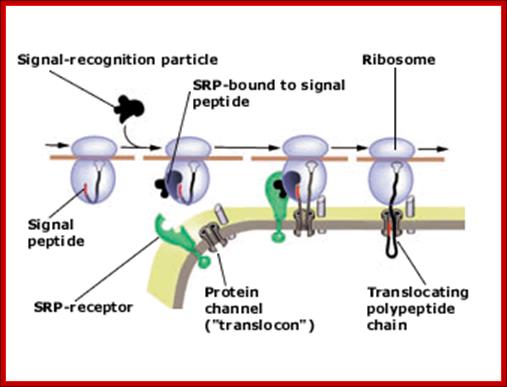
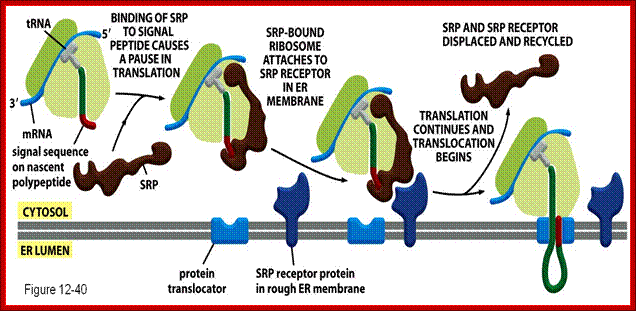
Figure: Cotranslational targeting of secretory proteins to the ER; Step 1: As the signal sequence emerges from the ribosome, it is recognized and bound by the signal recognition particle (SRP). Step 2: The SRP escorts the complex to the ER membrane, where it binds to the SRP receptor. Step 3: The SRP is released, the ribosome binds to a membrane translocation complex of Sec61 proteins, and the signal sequence is inserted into a membrane channel. Step 4: Translation resumes, and the growing polypeptide chain is translocated across the membrane. Step 5: Cleavage of the signal sequence by signal peptidase releases the polypeptide into the lumen of the ER ( NCB). http://oregonstate.edu/
As the signal sequence passes into the luminal side of ER, the signal peptidase (a five subunit protein) recognizes the signal segment and cuts it, and frees the signal fragments from main polypeptide chain. As the protein chain grows the N-terminal region of the protein is progressively drawn into the lumen. This movement is assisted by a class of proteins related to heat shock proteins (HSP) called BiP that uses ATP energy for the movement of polypeptide chain into the lumen.
- The protein that is drawn into the lumen is subjected to various post translational modifications, such as glycosylation, folding, S-S bond formation, trimming and other modifications. This happens as the protein is diffuses all along the lumen of ER. All the said processes of proteins are sequence specific and tissue specific. Destination of proteins is more or less pre determined and specific modification renders more specific.
- Those proteins, which fail to fold properly, are identified and marked and the same are transported back across the same translocon to cytosol for degradation; this is called retrograde transportation. Bip and Sec63 assist the translocon to translocate the lumen protein into cytoplasm where they are marked by ubiquitin and degraded by proteasomes. This translocation can be considered as one of the post-translational process.
Post-Translational free protein’s Transport:
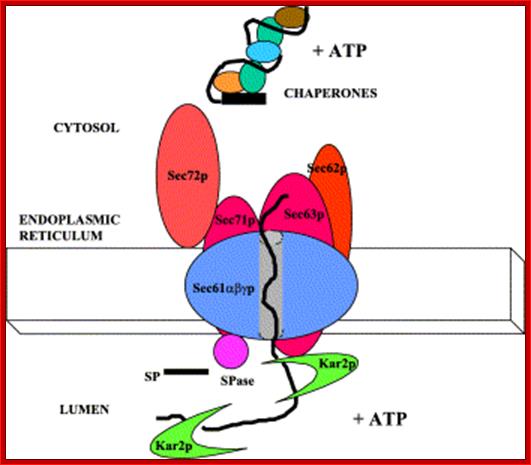
Posttranslational translocation of proteins into the ER: A cytosolic complex of molecular chaperones and ATP (+ATP) facilitate this pathway of protein translocation. The translocon consists of the Sec61α,β,γ proteins and Sec63p. Other proteins, Sec62p, Sec71p, and Sec72p, are also required for efficient protein translocation. Kar2p is the yeast homologue of GRP78 and is required to pull the substrate protein into the ER lumen concomitant with ATP hydrolysis (+ATP). The signal peptide (SP) at the amino terminus of substrate proteins is cleaved by signal peptidase (SPase).Traslocon.mol.Bio?
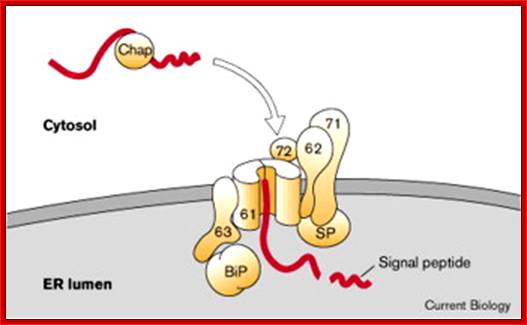
The post-translational translocation machinery of the yeast E:. The precursor protein is kept in a loosely-folded, translocation-competent conformation by its interaction with cytosolic chaperones (Chap). Targeting of the preprotein to the ER is thought to involve Sec62, Sec71 and Sec72. The core components of the translocon consist of Sec61, Sbh1 and Sss1, which associate to form a protein-translocating channel in the membrane (horseshoe-shaped structure). In the yeast ER lumen, the resident Hsp70 BiP is recruited to the trans side of the translocon by binding to Sec63. SP, signal peptidase.?
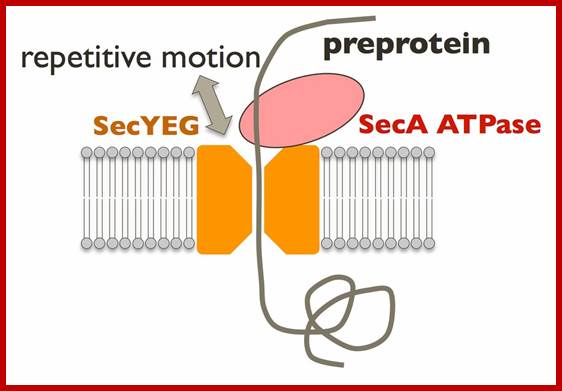
Novel measurement techniques; http://vsb.bmr.kyushu-u.ac.jp; Translocation of secretory proteins through the membrane is one of the evolutionally conserved mechanisms. Biological lipid bilayers generally prevent the passage of ions and small molecules. In order to achieve the transport of large molecules such as proteins, all cells possess well-controlled, specialized machineries for the secretion. We perform structural biological analyses for elucidation of protein translocation via SecYEG translocon, a conserved protein-conducting channel. Because the SecYEG complex is itself a passive channel, the driving force is required. SecA ATPase, a SecYEG-associated cytosolic motor, repeatedly pushes the substrate protein into SecYEG channel using the energy of ATP hydrolysis (Fig. left). It is one of the most important issues to fully understand the essential protein translocation. Hence, lots of functional and structural analyses have been reported since 1970's. From around 2000, X-ray crystal structures of Sec components have been solved one after another. Our group also has been determined the crystal structures of the bacterial Sec factors, SecYEG, SecA and SecDF. Although several researchers have been analyzing the molecular mechanism based on the crystal structures, the conformational changes and interactions of Sec proteins during the protein translocation still remains unclear. Therefore, to visualize dynamic protein translocation reaction we reconstituted one unit of Sec machinery in vitro using Nanodisc and are trying to image it by high-speed atomic force microscopy (AFM) (Fig. right). http://vsb.bmr.kyushu-u.ac.jp
Many proteins are released into cytoplasm in free-state from ribosomes. Such proteins are involved in metabolism such as glycolysis, fatty acid metabolism, amino acid turnover, protein turn over, carbohydrate turnover; some are cytoskeleton proteins required for maintaining cell shape, transport of mRNA and proteins even small organelles. On the other hand a large number of proteins as they exit from ribosomes are chaperoned and directed to specific cell organelles such as Mitochondria, vacuoles/ Lysosomes, Chloroplasts, Peroxisomes/Glyoxysomes and the Nucleus. They are guided by chaperones and also recognized by specific receptor proteins in sequence specific manner. The other proteins remain free in cytoplasm
Many other proteins are destined for secretion or certain other structures. The free proteins are bound and assisted by cytosolic chaperones to prevent aggregation. Some such proteins are transported into ER after the release of proteins in free-form. Such proteins’ translocation requires Sec 61 and four more proteins including BiP (Binding Protein like chaperones Hsps) to assist the channel forming proteins; ATP fuels the process. Specific proteins associate with such proteins using specific amino acid sequences and then they are threaded into a complex made up of Sec72, Sec71, Sec63, Sec62, Sec61 alpha, beta and gamma translocon complexes. The translocation into ER is ATP charged.
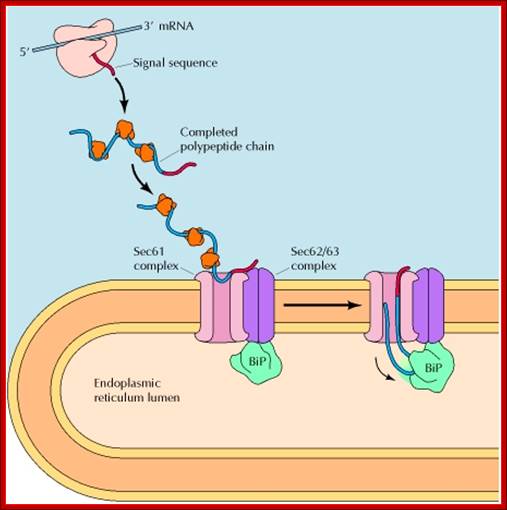
Figure: Posttranslational translocation of proteins into the ER: Proteins destined for posttranslational import to the ER are synthesized on free ribosomes and maintained in an unfolded conformation by cytosolic chaperones. Their signal sequences are recognized by the Sec62/63 complex, which is associated with the Sec61 translocation channel in the ER membrane. The Sec63 protein is also associated with a chaperone protein (BiP), which acts as a molecular ratchet to drive protein translocation into the ER (NCBI). http://biotechhelpline16.blogspot.in/
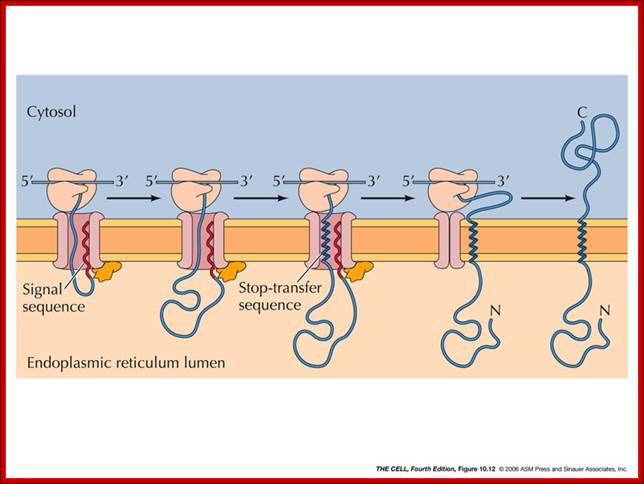
Depending upon the hydrophobic sequences the newly produced polypeptide chain anchors to the translocon and the rest is translocated, this results in anchoring the protein in ER membrane;http://oregonstate.edu/
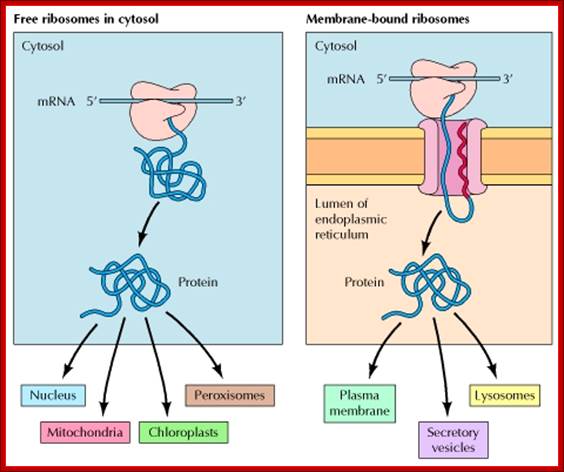
In mammalian cells, the initial sorting of proteins to the ER takes place while translation is in progress. Proteins synthesized on free ribosomes either remain in the cytosol or are transported to the nucleus, mitochondria, chloroplasts, or peroxisomes. In contrast, proteins synthesized with hydrophobic signal sequences on membrane-bound ribosomes are translocated into the ER while their translation is in progress. They may be either retained within the ER or transported to the Golgi apparatus and, from there, to lysosomes, the plasma membrane, or the cell exterior via secretory vesicles, NCBI; te.eduoregonsta.
Protein folding - the role of Chaperones and Chaperonins:
Once polypeptide chain is threaded into sec channel into the lumen, it is guided by ER lumen chaperones. Many of them are modified in terms of Glycosylation and such other modifications. This leads to proper folding and stabilized by S-S bond formation.
Disulphide bond formation aided by specific sequences; it is one of the most important features of Protein folding into its 3-D structural form; this requires site specific modification of nascent polypeptide chain such as glycosylation. Disulfide bond formation is facilitated by Glutaredoxin and Thioredoxin; both pathways are more or less similar.
https://pubchem.ncbi.nlm.nih.gov
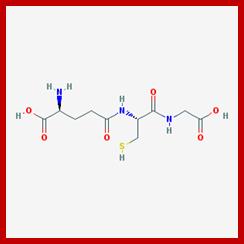
L-glutamic acid, L-cysteine and glycine components of Glutathione.
Glutathione is a tripeptide with many roles in cells. It conjugates to drugs to make them more soluble for excretion, is a cofactor for some enzymes, is involved in protein disulfide bond rearrangement and reduces peroxides.
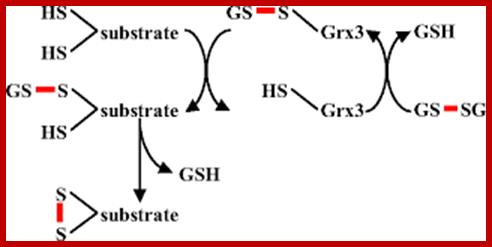
Glutaredoxin (Grx)
Model for disulfide bond formation by a monothiol Glutaredoxin, adopted from (19). A glutathionylated Glutaredoxin is attacked by a substrate cysteine, producing reduced Glutaredoxin and glutathionylated substrate. In a second step, a second cysteine within the substrate attacks the glutathione-substrate disulfide, resulting in oxidized substrate.
A) Glutaredoxin (Grx) catalyses the reduction of disulfide bonds in proteins converting glutathione (GSH) to glutathione disulfide (GSSG). GSSG is in turn recycled to GSH by the enzyme glutathione reductase at the expense of NADPH. During the reaction cycle it is thought that a cysteine pair in the active site of glutaredoxin is converted to a disulfide. B) Glutaredoxin is also thought to be important for deglutathionylation of protein thiols. In this reaction only a single cysteine is required. Indeed, many naturally occurring glutaredoxins contain only one cysteine in the active site. It should be noted that the direction of the glutaredoxin-catalyzed cycle depends on the relative concentrations of GSH and GSSG. High concentrations in the cell of GSSG relative to GSH will drive glutathionylation or the oxidation of protein thiols to disulfides.
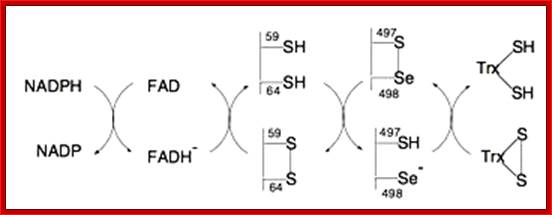
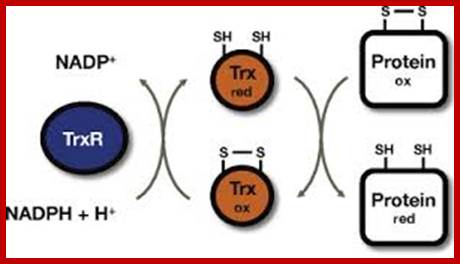
Thioredoxin (TRX); The manipulation of cellular redox status has emerged as a promising therapeutic strategy to prevent uncontrolled inflammatory response. Thioredoxin is an important regulator of cellular redox homeostasis, which catalyzes the reduction of disulfide bonds. Human thioredoxin, originally identified as a secretory protein ADF, has been implicated in a wide variety of redox regulations in both intracellular and extracellular compartments. This review includes a summary of the evidence available supporting the employment of the beneficial properties of thioredoxin to combat inflammation, an evaluation of the potential of redox-based therapy for the treatment of inflammatory diseases, and a discussion on the conceptual model of a redox-sensitive signaling complex, Redoxisome, consisting of thioredoxin and its redox partners Yoahlyki Matsu; http://www.cgfr.co.uk
Role of Calnexin and Calreticulin Action in protein folding:
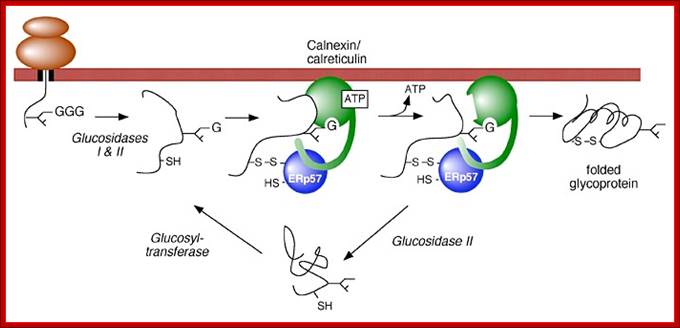
Role of Calnexin and Calreticulin in guiding protein that is entered into ER for folding and S-S bond formation; http://biochem17.med.utoronto.ca/
Model of calnexin and calreticulin action. As a nascent polypeptide chain enters the ER, certain Asn residues are glycosylated through the addition of an oligosaccharide of composition Glc3Man9GlcNAc2. The outermost two glucoses are rapidly removed through the action of glucosidases I and II to reveal the monoglucosylated species recognized by the lectin sites of calnexin/calreticulin. In their ATP-bound state, calnexin and calreticulin bind to the monoglucosylated oligosaccharide (via their lectin sites) as well as to hydrophobic segments of the unfolded glycoprotein (via their polypeptide binding sites). Glycoprotein dissociation involves not only the action of glucosidase II to remove the terminal glucose residue but also a change in affinity of the polypeptide binding site, possibly regulated by a shift from an ATP-bound to an ADP-bound or unbound state. After dissociation, if folding does not occur rapidly, the glycoprotein is reglucosylated by another ER enzyme, UDP-glucose:glycoprotein glucosyltransferase. This enzyme only reglucosylates non-native protein conformers. The glycoprotein can then re-bind in dual fashion to the ATP form of calnexin/calreticulin. In this model, both the glucosyltransferase and calnexin/calreticulin act as folding sensors. The function of this binding and release cycle is three-fold: 1) the polypeptide binding site prevents glycoprotein aggregation, 2) the lectin and polypeptide sites retain non-native conformers in the ER until a native structure is acquired (quality control), and 3) calnexin and calreticulin bring ERp57 or cyclophilin B into proximity with the non-native glycoprotein to catalyze disulfide formation/isomerization or proline isomerization reactions, respectively. Williams Lab; http://biochem17.med.utoronto.ca/
Calnexin (CLN) and Calreticulin (CRT) are lectins. Calnexin is bound to luminal side of ER membrane and CRT is free from the membrane. As a nascent polypeptide chain enters the ER lumen, certain Asn residues are glycosylated through the addition of an oligosaccharide group consisting of Glc3Man9GlcNAc2. This happens even before the proteins are folded into proper shape. The outermost two glucoses of oligosaccharide group are rapidly removed through the action of glucosidases I and II to reveal the monoglucosylated species recognized by the lectins called calnexin/calreticulin. In their ATP-bound state, calnexin and calreticulin bind to the monoglucosylated oligosaccharide (via their lectin sites) as well as to hydrophobic segments of the unfolded glycoprotein (via their polypeptide binding or chaperone sites). Glycoprotein dissociation involves not only the action of glucosidase II to remove the terminal glucose residue but also a change in affinity of the polypeptide binding site, possibly regulated by a shift from an ATP-bound to an ADP-bound or unbound state. After dissociation, if folding does not occur rapidly, the glycoprotein is glycosylated by another ER enzyme, UDP-glucose: glycoprotein glucosyltransferase. The said enzymes perform glycosylation only non-native protein conformers. The glycoprotein can then re-bind in dual fashion to the ATP form of calnexin/calreticulin. In this model, both the glucosyl transferase and calnexin/calreticulin act as folding sensors. The function of this binding and release cycle is three-fold: 1) the polypeptide binding (chaperone) site prevents glycoprotein aggregation, 2) the lectin and polypeptide sites retain non-native conformers in the ER until a native structure is acquired (quality control), and 3) calnexin and calreticulin bring ERp57 into proximity with the non-native glycoprotein. ERp57 catalyzes disulfide bond formation and isomerization within the glycoprotein substrate via a mixed disulfide intermediate involving a substrate cysteine and cysteines within the active site -CGHC- motifs of ERp57 (-S-S-).
Calreticulin (CTR or CALR):
Cal reticulin is free from ER membrane. Calreticulin binds to misfolded proteins and prevents them from being exported from the Endoplasmic reticulum to the Golgi apparatus. CALR is a multifunctional protein that acts as a main Ca (2+)-binding (storage) protein in the lumen of the endoplasmic reticulum. Calreticulin that is localized in the nucleus participates in transcription regulation. Human recombinant protein-CTR’s molecular weight is 48.7Kda.
A similar quality-control chaperone, Calnexin, performs the same service for soluble proteins as doe’s calreticulin. Both proteins, Calnexin and Calreticulin, have the function of binding to oligosaccharides containing terminal glucose residues. In normal cellular function, trimming of glucose residues off the core oligosaccharide added during N-linked glycosylation is a part of protein processing. If "overseer" enzymes note that residues are misfolded, proteins within the RER will re-add glucose residues so that other Calreticulin/Calnexin can bind to these proteins and prevent them from proceeding to the Golgi. This leads these aberrantly folded proteins down a path whereby they are targeted for degradation. Studies on transgenic mice reveal that calreticulin is cardiac embryonic gene that is essential during development.
Calnexin (CNX):
It is a 90kDa integral protein of the endoplasmic reticulum (ER) bound to inner surface of the ER by transmembrane domain. It consists of a large (50 kDa) N-terminal calcium-binding luminal domain, a single transmembrane helix and a short (90 residues), acidic cytoplasmic tail. Calnexin is one of the chaperone molecules, which are characterized by their main function of assisting protein folding and quality control, ensuring that only properly folded and assembled proteins proceed further along the secretory pathway. The function of calnexin is to retain unfolded or unassembled N-linked glycoproteins in the endoplasmic reticulum. Calnexin binds only those N-glycoproteins that have GlcNAc2Man9Glc1 oligosaccharides. Oligosaccharides with three sequential glucose residues are added to asparagine residues of the nascent proteins in the ER.
The monoglucosylated oligosaccharides that are recognized by calnexin result from the trimming of two glucose residues by the sequential action of two glucosidases, I and II. Glucosidase II can also remove the third and last glucose residue. If the glycoprotein is not properly folded, an enzyme called UGGT (for UDP-glucose: glycoprotein glucosyl transferase) will add the glucose residue back onto the oligosaccharide thus regenerating the glycoprotein's ability to bind to calnexin. The improperly-folded glycoprotein chain thus loiters in the ER, risking the encounter with MNS1 (alpha-mannosidase), which eventually sentences the underperforming glycoprotein to degradation by removing its mannose residue. If the protein is correctly translated, the chance of it being correctly folded before it encounters MNS1 is high. ATP and calcium ions are two of the cofactors involved in substrate binding for calnexin. Calnexin also functions as a chaperone for the folding of MHC class I alpha chain in the membrane of the ER. After folding is completed Calnexin is replaced by Calreticulin, which assists in further assembly of MHC class I.
Chaperones:
We define a chaperone as any protein that interacts, stabilizes, or helps a non-native protein to acquire its native conformation, but is not present in the final functional structure. Chaperones are involved in a multitude of cellular functions, including de novo folding, refolding of stress-denatured proteins, oligomeric assembly, intracellular protein transport, and assistance in proteolytic degradation. The chaperones, that participate broadly in protein biogenesis, such as the Hsp70s and chaperonins (Hsp60s), promiscuously recognize hydrophobic amino acid side-chains exposed by non-native proteins and promote folding through ATP-regulated binding and release cycles. Chaperone binding blocks aggregation, whereas transient release of bound hydrophobic regions is necessary for folding to proceed. It is important to realize that chaperones act not by contributing steric information to the folding process, but rather by optimizing the efficiency of folding. Notably, a number of essential proteins, such as actins and tubulins, encounter high energetic barriers in folding and are unable to reach their native states in the absence of chaperones. Their folding is mediated by barrel-shaped chaperonins, a specialized chaperone class. Because mutations often disrupt a protein’s ability to fold, it follows that the chaperone system is also important in buffering such deleterious mutations. This buffering function is thought to be critical in the evolution of new protein functions and phenotypic traits, (Maisnier-Patin et al. 2005; Tang et al. 2006). (Rutherford and Lindquist 1998; Kerner et al. 2005; Maisnier-Patin et al. 2005; Tokuriki and Tawfik 2009), (Hartl 1996; Hartl and Hayer-Hartl 2009.
RNA Chaperones:
Chaperones are not just restricted to proteins, there are chaperones which bind and fold RNAs into their proper structures. RNA chaperones are ubiquitous, heterogeneous proteins essential for RNA structural biogenesis and function. We investigated the mechanism of chaperone-mediated RNA folding by following the time-resolved dimerization of the packaging domain of a retroviral RNA at nucleotide resolution. In the absence of the nucleocapsid (NC) chaperone, dimerization proceeded via multiple, slow-folding intermediates. In the presence of NC, dimerization occurred rapidly via a single structural intermediate.
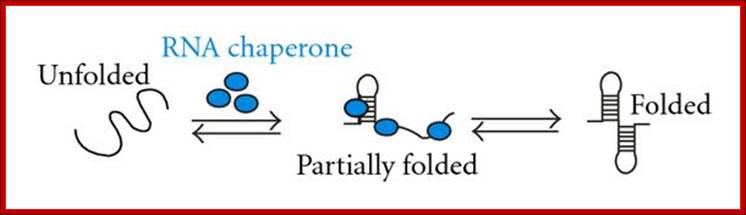
Hypothetical mechanisms of RNA chaperoning. (a) shows folding of an RNA molecule in the presence of RNA chaperones (blue). RNA chaperones and proteins with RNA chaperone activity prevent the RNA from misfolding and increase annealing of the correct structure by crowding. (b) Proteins with RNA chaperone activity possessing disordered regions (blue) interact with misfolded RNA. Upon energy transfer, the RNA structure loosens and the disordered protein domain becomes more ordered. Proteins with RNA chaperone activity are dispensable in both cases after the RNA has folded into its native form. http://www.hindawi.com/
The RNA binding domain of hnRNP A1 protein (UP1), a structurally unrelated chaperone, also accelerated dimerization. Both chaperones interacted primarily with guanosine residues. Replacing guanosine with more weakly pairing Inosine yielded an RNA that folded rapidly without a facilitating chaperone. These results show that RNA chaperones can simplify RNA folding landscapes by weakening intramolecular interactions involving guanosine and explain many RNA chaperone activities; Grohman JK, Gorelick RJ, Lickwar CR, Lieb JD, Bower BD, Znosko BM, Weeks KM.
From the cradle to the grave: molecular chaperones that may choose between folding and degradation; Jörg Höhfeld, Douglas M. Cyr & Cam Patterson
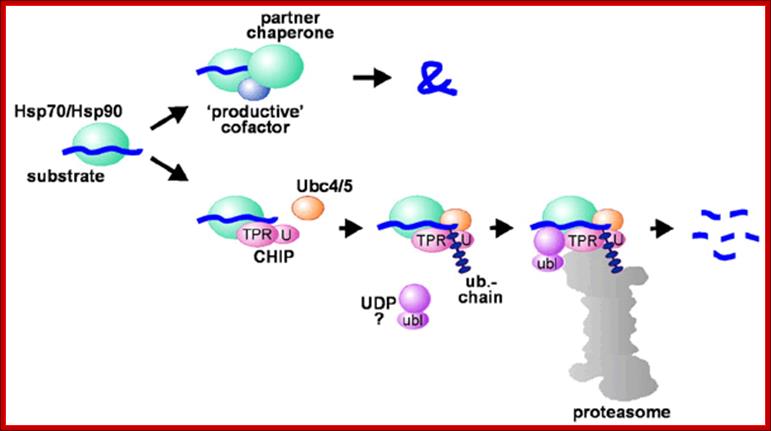
Hsp70 and Hsp90 in protein folding and degradation; An initial decision to fold or degrade a Hsp70- or Hsp90-associated substrate protein may be reached through competition between a 'productive' cofactor such as Hop (cochaperone) (Hsc70-Hsp90 organizing protein) and the ubiquitin ligase CHIP (C-terminus of Hsc70-interacting protein). During the folding process, Hsp70 and Hsp90 may co-operate with other chaperone proteins (termed partner chaperones). On the degradation pathway, CHIP associates with Hsp70 or Hsp90 via its TPR chaperone adaptor (TPR), and at the same time recruits E2 ubiquitin conjugating enzymes of the Ubc4/5 family to the chaperone complex. This may involve binding of the E2 to the U-box of the cofactor (U). In conjunction with E2, CHIP mediates ubiquitin attachment to the chaperone substrate and induces its targeting to the proteasome for degradation. The targeting process may be facilitated by a ubiquitin domain protein (UDP), such as BAG-1, which binds to Hsp70 and utilizes its ubiquitin-like domain (ubl) for proteasomal association; http://embor.embopress.org/
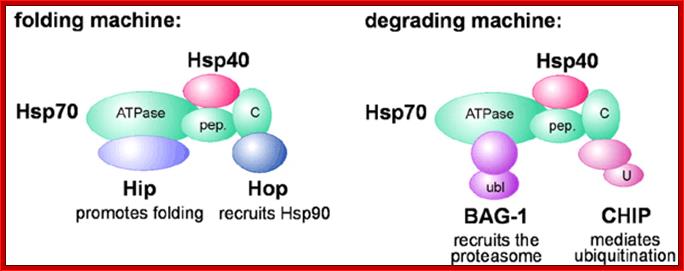
Model for Hsp70 chaperone machines. Binding of distinct chaperone cofactors to the N‐terminal ATPase domain of Hsp70 (ATPase) and to its C‐terminus (C) may give rise to chaperone machines involved in protein folding and protein degradation, respectively. The cofactors Hip and BAG‐1 compete for binding to the ATPase domain, while Hop and CHIP associate with the C‐terminus in a competitive manner. During folding and degradation, Hsp70 appears to co‐operate with cofactors of the Hsp40 protein family. pep., peptide binding domain of Hsp70; ubl, ubiquitin‐like domain of BAG‐1; U, U‐box of CHIP. http://embor.embopress.org/
The Chaperonins:
Chaperonins are chaperones and they are large double-ring complexes of approximately 800 kDa. Two groups of chaperonin exist (Horwich et al. 2007; Tang et al. 2007): Group I chaperonins (also called Hsp60s) occur in bacteria (GroEL), mitochondria, and chloroplasts. They have seven-membered rings and functionally cooperate with Hsp10 proteins (GroES in bacteria), which form the lid of the folding cage.
The group II chaperonins in archaea (thermosome) and in the eukaryotic cytosol (TRiC/CCT) have eight- or nine-membered rings. They are independent of Hsp10 factors, as their lid function is built into the chaperonin ring in the form of specialized α-helical extensions. Unlike the Hsp70s, the chaperonins promote folding through ATP-regulated cycles of global protein encapsulation. Horwich et al. 2007; Tang et al. 2007
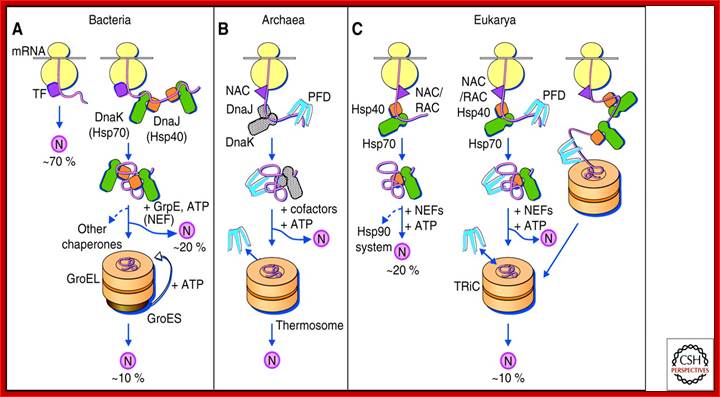
Protein folding in the cytosol. (A) Models for the chaperone-assisted folding of newly synthesized polypeptides in the cytosol. Bacteria (left panel). TF, trigger factor; N, native protein. Nascent chains probably interact generally with TF, and most small proteins (∼70% of total) may fold rapidly on synthesis without further assistance. Longer chains interact subsequently with DnaK and DnaJ (Hsp70 system) and fold on one or several cycles of ATP-dependent binding and release (∼20% of total). About 10% of chains transit the chaperonin system (GroEL and GroES) for folding. (B) Archaea (middlepanel). PFD, prefoldin; NAC, nascent chain-associated complex. Note that only some archaeal species contain DnaK/DnaJ. (C) Eukarya (right panel). Like TF, NAC (nascent chain associated complex) probably interacts generally with nascent chains, but the role of NAC in folding is not yet clear. About 20% of chains reach their native states in a reaction assisted by RAC (ribosome associated complex), Hsp70, and Hsp40. A fraction of these must be transferred to Hsp90 for folding. About 10% of chains are co- or posttranslationally passed on to the chaperonin TRiC in a reaction mediated by Hsp70 and PFD, both of which interact directly with TRiC. PFD recognizes the nascent chains of certain TRiC substrates, including actin and tubulins. (Modified, with permission of The American Association for the Advancement of Science, from; Hartl and Hayer-Hartl 2002.);http://cshperspectives.cshlp.org/
Protein folding in the cytosol:
(A) Models for the chaperone-assisted folding of newly synthesized polypeptides in the cytosol: Bacteria (left panel). ( TF- trigger factor; N, native protein). Nascent chains probably interact generally with TF, and most small proteins (∼70% of total) may fold rapidly on synthesis without further assistance. Longer chains interact subsequently with DnaK and DnaJ (Hsp70 system) and fold on one or several cycles of ATP-dependent binding and release (∼20% of total). About 10% of chains transit the chaperonin system (GroEL and GroES) for folding.
(B) Archaea (middle panel): PFD (prefoldin); NAC (nascent chain-associated complex). Note that only some archaeal species contain DnaK/DnaJ.
(C) Eukarya (right panel). Endoplasmic reticulum lumen resident BiP proteins are resident chaperonins. General chaperones: BiP, GRP94, GRP170. Lectin chaperones: calnexin and calreticulin, Non-classical molecular chaperones: HSP47 and ERp29, Folding chaperones: Protein disulfide isomerase (PDI), Peptidyl prolyl cis-trans-isomerase (PPI), ERp57
Like TF, NAC (nascent chain associated complex) probably interacts generally with nascent chains, but the role of NAC in folding is not yet clear. About 20% of chains reach their native states in a reaction assisted by RAC (ribosome associated complex), Hsp70, and Hsp40. A fraction of these must be transferred to Hsp90 for folding. About 10% of chains are co- or post-translationally passed on to the chaperonin TRiC in a reaction mediated by Hsp70 and PFD, both of which interact directly with TRiC. PFD recognizes the nascent chains of certain TRiC substrates, including actin and tubulins; Hartl and Hayer-Hartl 2002.
Chaperone paradigms: Chaperonins and Hsp90 system.
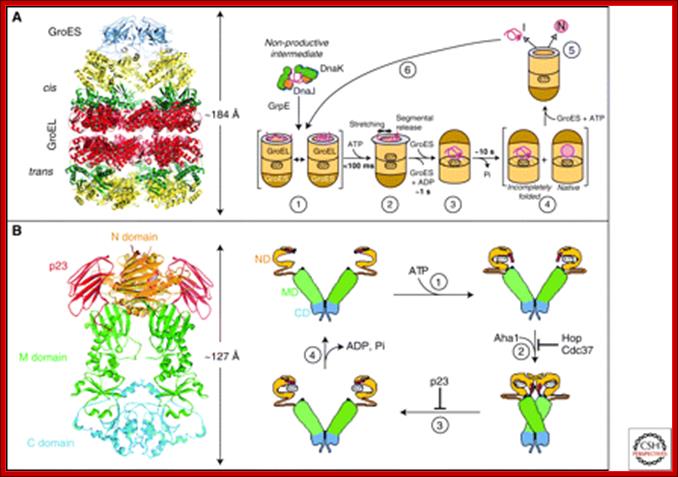
(A) The GroEL-GroES chaperonin. Left panel: Crystal structure of the asymmetric GroEL-GroES complex (PDB 1AON) (Xu et al. 1997). The figure below shows Cis-the GroES-bound chamber of GroEL and Trans, the opposite GroEL ring. Right panel: Working model summarizing the conformational changes in a substrate protein on transfer from DnaK/DnaJ (Hsp70 system) to GroEL and during GroEL/GroES-mediated folding. Note that binding of a second substrate molecule to the open ring of GroEL in steps 4 and 5 as well as the transient formation of a symmetrical GroEL-GroES2 complex is omitted for simplicity. (1) Substrate protein may be delivered to GroEL by DnaK/DnaJ in a non-aggregated, but kinetically trapped state. On binding to GroEL it undergoes local unfolding to an ensemble of expanded and more compact conformations. (2) ATP-dependent domain movement of the apical GroEL domains result in stretching of tightly bound regions of substrate and in release and partial compaction of less stably bound regions. (3) Compaction is completed on substrate encapsulation by GroES. (4) Folding in the chaperonin cage. (5) Substrate release on GroES dissociation. (6) Rebinding of incompletely folded states. N, native state; I, folding intermediate; from Hartl and Hayer-Hartl 2009.
(B) The Hsp90 system. Left panel: Crystal structure of the Hsp90 dimer from S. cerevisiae with the regulator p23 bound to the amino-domain (PDB 2CG9) (Ali et al. 2006). Right panel: ATPase cycle of Hsp90. On ATP binding (1) the amino-terminal ATPase domain (ND) of Hsp90 undergoes a conformational change leading to the closure of the ATP lid. After lid closure, the first 24 amino acid residues of each Hsp90 monomer dimerize and the first β-strand and α-helix swap to associate with the ND of the other monomer (2). Furthermore, in each monomer, the ND contacts the corresponding M-domain (MD). This metastable conformation is committed for ATP hydrolysis (3). This results in a compaction of the Hsp90 dimer, in which the individual monomers twist around each other. After hydrolysis (4), the NDs dissociate and both monomers separate amino-terminally. Various cofactors regulate this cycle: Cdc37, which delivers certain kinase substrates to Hsp90, inhibits the ATPase activity, and Hop, a TPR-protein that joins Hsp70 to Hsp90, inhibits ND dimerization. Aha1 stimulates ATP hydrolysis, whereas p23 stabilizes the dimerized form of Hsp90 before ATP hydrolysis. These factors are thought to adjust the kinetic properties of the cycle to achieve certain conformational transitions in Hsp90-bound client proteins, as well as their release from Hsp90. (Modified, with permission, from Wnadinger et al 2008.
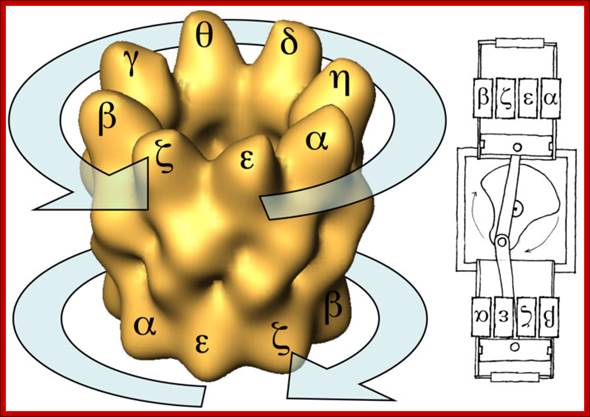
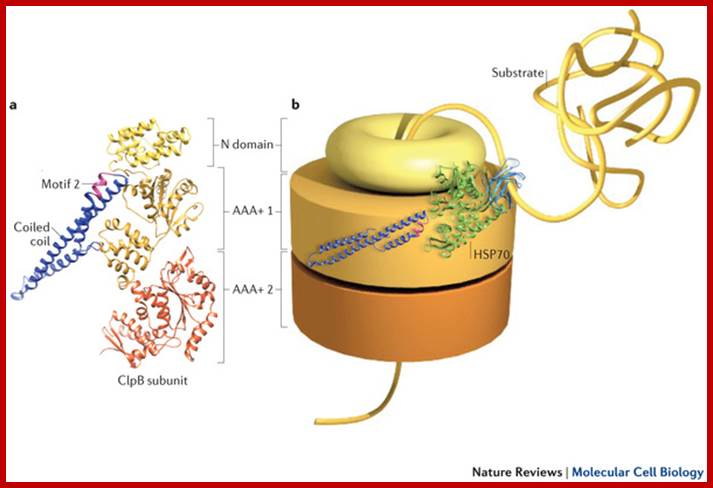
Chaperone- made up of two eight protein subunit rings from alpha to, nano (micro), delta, theta, gamma, beta, chi, epsilon; superimposed as barrel, subunits are in opposite orientation. Molecular chaperones are diverse families of multidomain proteins that have evolved to assist nascent proteins to reach their native fold, protect subunits from heat shock during the assembly of complexes, prevent protein aggregation or mediate targeted unfolding and disassembly. Their increased expression in response to stress is a key factor in the health of the cell and longevity of an organism. Unlike enzymes with their precise and finely tuned active sites, chaperones are heavy-duty molecular machines that operate on a wide range of substrates. The structural basis of their mechanism of action is being unravelled (in particular for the heat shock proteins HSP60, HSP70, HSP90 and HSP100) and typically involves massive displacements of 20–30 kDa domains over distances of 20–50 Å and rotations of up to 100°. http://www.nature.com
It is becoming more and more evident that the cellular functions are performed by proteins that work in a coordinated manner to perform a given biological process in a more efficient manner. One of such processes is the protein folding pathway, controlled by a group of proteins termed molecular chaperones, which assist the folding of other proteins. We plan to study some of these chaperones, in particular the eukaryotic chaperonin CCT and its archaeal counterpart, the thermosome, using as a main tool electron microscopy and image processing.
Orientation of Protein’s Amino and Carboxyl Termini Anchored to Membranes:
Position and the number of signal sequences and the position and the number of charged or uncharged sequences in the protein, ultimately determines the orientation of proteins’ N-terminal and C-terminal ends with respect to membrane surfaces i.e. cytosolic and exoplasmic. It is important to understand the outer (exoplasmic) and inner (cytosolic) surface of a membrane. Orientation and anchoring of single pass or multipass proteins takes place during co translation process.
· The endoplasmic reticulum, which can exist as two membrane flat sheets or two membranes extended in the form of flat sheets or tubules or vesicles; all have an internal luminal space filled with fluid; this space in the case of ER flat membranes or tubules is termed as ER-lumen, whose character and content is more or less equivalent to exterior or exoplasmic, so the membrane facing lumen is equivalent to exoplasmic surface and the other side is cytosolic surface. The luminal space filled with a variety of proteins that performs specific functions in protein transport and modifications.
· The space found between the outer and inner membranes in mitochondria, chloroplasts, golgi complex and the nucleus is same as exoplasmic. The fluid found in Lysosome, Endosomes, Phagosome, Peroxisomes and vesicles from endocytosis are more or less similar to that that of exoplasmic space, though their content may vary in each of the cases.
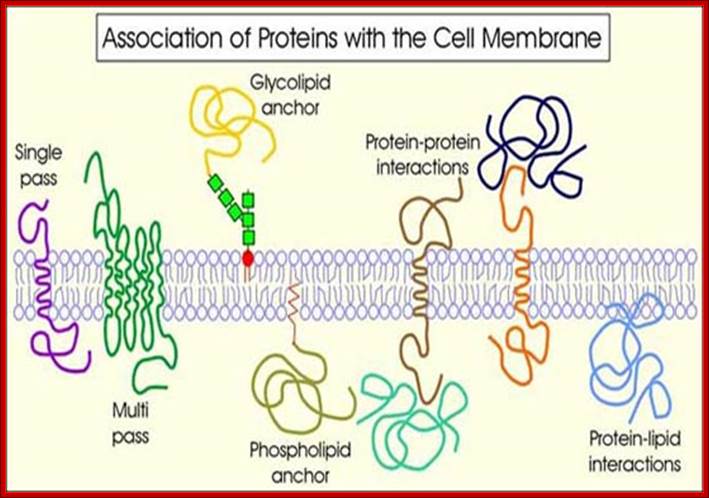
Proteins may contain a single lipid-spanning domain (single pass) or several (multipass). They may be linked to the membrane by a glycolipid or phospholipid anchor. Proteins that are linked to or embedded in the cell membrane may associate with other proteins (protein-protein interactions) either on the inner or outer face of the membrane. Proteins may interact directly with lipids in the bilayer. http://www.nfsdsystems.com/
Orientation of polypeptide ends with respect to cytosolic and exoplasmic sides of the membrane take place while the protein is synthesized on ER and transported into it. If the N-terminal ends are made up of signal sequences, automatically the N-terminal of the protein will be towards endoplasmic/exoplasmic space. While the protein chain is elongating without any N-terminal signal sequences and after a length of polypeptide chain elongation, a sequence of hydrophobic amino acids are generated; then the SRPs binds to such sequences and load the hydrophobic region onto translocon and the protein elongation continues. The hydrophobic sequences as they generate helix structures they are transferred into membranes, they are held there, but the continued synthesis of protein makes the segment lie towards exoplasmic side; this continues till another stretch of hydrophobic sequences are added the growing polypeptide chain. Then this hydrophobic region is held on to the membrane and next stretch of amino acids added will be dispensed towards cytosolic side. This is how multipass membrane anchoring proteins (TM) are produced. Heat shock proteins (HSP) called BiP that uses ATP energy is used for the movement of polypeptide chain into the lumen.
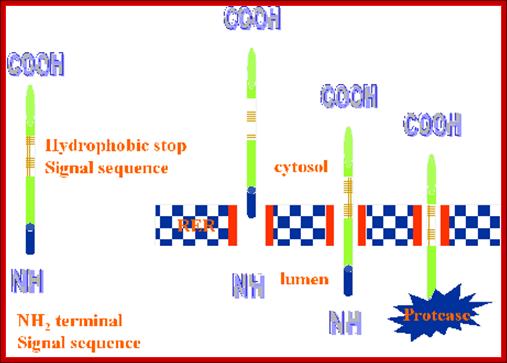
This is a basic diagram showing the passage of protein chains and stop at a position called anchor sequences i.e. anchor to the membrane. http://www.cytochemistry.net/
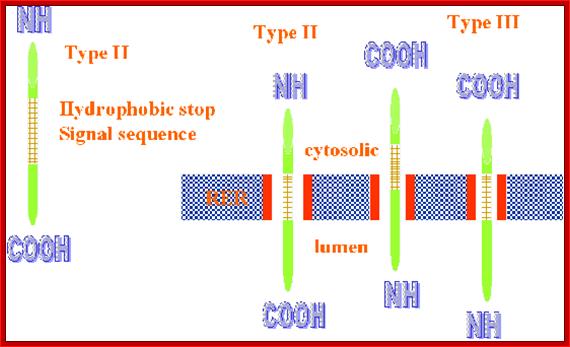
Based on the orientation of protein with respect to their N or C ends of polypeptide chains they are classified into different types; the above diagram shows Type II and Type III; http://www.cytochemistry.net/
· Type-I: Transmembrane proteins, are the major number of proteins belongs to this category, consists of C- terminal region at cytosolic surface of the plasma membrane and the rest of the protein is at extra cellular surface. In this kind of disposition of the protein, the N-terminal signal sequences are cleaved. It has stop transfer hydrophobic region (22 aa) in the middle of the protein. At the Cytosolic side of the stop transfer region one finds positive charges amino acids, which bind to negative charged phospholipids of the membranes. Ex. Glycophorin, LDL receptor, Influenza HA protein, Insulin receptor, growth hormone receptors.
· Type-II: In type -II proteins, the whole N-terminal region is at cytoplasmic side and a short C- terminal region at outer surface of the membrane; in such cases the signal sequences are not cleaved and they are found at cytosolic regions. Such proteins are not as abundant as the Type-I proteins, Ex. glycoproteins receptor, Transferrin receptor, Golgi galactosyl transferase, Golgi sialyl transferase, and Influenza HN protein and others.
Another class II have short exoplasmic N-terminal and long C-end at cytoplasmic segment, they don’t have cleavable N-signals instead contain one stop transfer signal, which also acts as anchor sequence ex.; cytochrome-C.
· Type III: Both sides are equally distributed to cytosolic and exoplasmic sides.
· Type IV: Multipass proteins. Multiple pass proteins have many hydrophobic helical regions as stop transfer sequences and positively charged amino acids just before helical regions at Cytosolic side, they firmly hold proteins in the membrane. Based on the number of stop transfer sequences they are classified into many types, such as Type I to type IV (the fourth has IVA and IVB). The first type has internal stop transfer anchor sequences (STA), others have internal signal anchor sequences (SA II and SA III).
· Other types: They don’t have hydrophobic membrane spanning segment, but they are anchored to amphipathic phospholipid anchors embedded in the membranes.
Multipass anchored membrane proteins and others shown ;http://themedicalbiochemistrypage.org/
· Many multipass proteins are found to be receptor on cell surface. Similarly, many multipass proteins act as channels either with hydrophilic surfaces or hydrophobic surfaces towards the channel space.
· Multipass proteins need not be single protein, but can be associated with two or more of the same or different proteins.
· That does not fix the protein in a particular position in the membrane, for all proteins, both integral and extrinsic proteins and all phospholipids and other lipids or lipid like molecules will be in constant motion by what is termed as lateral diffusion.
· Depending upon where the linking compounds present and kind of proteins that linked and the kind of membrane or organelles involved, their orientation can be towards exterior or they can be at cytosolic surface or they can be buried in the membrane.
In many cases the proteins, either at N-terminal region or at C-terminal region, are linked to lipid soluble membrane components such as Farnesyl molecules (15C), Geranyl (10C) components (isoprene derivatives) or Steroyl (18C) or Myristoyls (14C) or Palmitoyl (16C) and in many cases proteins are attached to membrane anchored to GPI (Glycosyl Phosphotidyl Inositol complex).
· Myristoyl (14C) COO^- group: It can be linked to an amino terminal of a protein; a myristoylated protein is mostly localized at cytosolic surface.
· Palmitoyl (16C) COO^- group: It can be linked to the OH group of any one of the internal serine or threonine of proteins, again localized at cytosolic surface.
· Steroyl (18C) COO^-: It can be bonded to SH group of any one of the internal cysteine and the protein is localized at cytosolic surface.
· Farnesyl (15C): It can be linked to cysteine at carboxy terminal of a protein (localized at cytosolic surface).
The above said lipid soluble components act as anchors for proteins, some of which may be disposed to the outer surface and some towards cytosolic surface. Specific enzymes perform these linkages. Most of the cellular lipids are synthesized at the cytosolic surface of ER and the enzymes responsible for synthesis or localized or anchored to membranes and their enzymes are at cytosolic surface.
Proteins disposed with N-end outer and C-end inner surface:
· VSV-Glyco protein,
· Glycophorin,
· LDL receptor,
· Influenza HA- protein (hemato-agglutinin protein)
· Insulin receptor protein.
Proteins with N-terminal inner and C-end outer surface:
· Transferrin receptor protein,
· Sucrose-isomaltose receptor protein,
· Golgi-galactosyl transferase protein,
· Golgi sialyl transferase protein,
· Influenza HN protein.
Single pass protein with N-terminal at the outer surface:
· Cytochrome B5.
Single pass protein with C-end at outer surface:
· Cytochrome p450.
Multiple membrane pass proteins:
Glucose transport protein with both N and C ends at inside surface.
Bacteriorhodopsin, beta adrenogenic receptor, Rhodesian (all have N-end at outside and c- terminal at inside.
Cellular sites associated with protein synthesis and transport:
Proteins that are synthesized on the ER as polysomes are transported into ER and assessed for overall quality. Folding is facilitated by interaction of the nascent polypeptide with molecular chaperones. Misfolded and misassembled products are retained in the ER and exposed to resident chaperones to attempt folding. Eventually, misfolded proteins are transported back to the cytoplasm for proteasomal degradation. Alternatively, defective proteins may be exported to and retained by the Golgi apparatus then retrotranslocated to the ER where correct folding is again attempted, or diverted to lysosomes via export out by ERAD for degradation. Mature products are then exported to their final destination (the PM). Pharmacoperones can frequently rescue misfolded proteins by correcting folding and allowing them to escape retention by the QCS and route to the plasma membrane where they are able to bind ligand and couple to the effector system.
Some of the proteins such as histones, glycolytic proteins, actins, tubulins and transcription factors and translation factors are released into cytosol. There are many such proteins that reside free in cytosol. A large number of proteins set free into cytosolic milieu are transported into the nucleus. Examples-Transcription factors and Histones.