Molecular Tools-I:
1.� Labeling Compounds:
Radioactive isotopes:
Use and Application:
Biological systems can be observed under microscope, which has been done in 18th century and most part of 19th century.� But they found it not enough to understand the biological processes and their molecules, so methods required are different and one such tool was the use of radioactive isotopes as tracer molecules, which can be employed to find out the pathway of a biochemical reaction or to identify the product and quantify the same or the target of the compound used.�
The use of radioactive isotopes has become an absolute necessity to confirm the results of biochemical processes and also used in SEM and TEM techniques.� Even today for very contrast and good results radioactive isotopes are preferred labels.� Autoradiograph of radioactive isotope labeled proteins, RNA and DNA is still best and preferable.� However with time people realized the hazards of the use of radioisotopes for some of the radioisotopes used such as gamma labeled Iodine, 32-P labeled, and 35S- labeled compounds emit beta radiation and in larger doses they can create health problems in long term or short term uses.� They are very short lived and found to be very expensive.�
Research scholars are as innovative as they ever found alternative tracer methods such as chromogenic or flourogeneic and luminogenic compounds, which are as good as radioactive isotopes which are user friendly and non-hazardous and they can be stored under proper conditions and be kept for longer duration without degradation or loss of the quality.� Some time some of these compounds are expensive they are still favored.
The most common radioactive isotopes used:
14* C for proteins and nucleic acids.
Alpha or gamma labeled dNTPs or rNTPs.
Labeled 131* Iodine for labeling proteins.
35*S, methionine and dATP.
Half-life of radioactive isotopes:
14*C- beta emission- half-life 5730 yrs,
3*H- soft beta emission- half-life-12.3 yrs,
32*P- hard beta emission- half-life- 14.3 days,
35*S- hard beta emission-half-life- 87.4 days,
125* I- gamma emission-half-life - 60 days,
131* I - gamma emission- half-life- 8.06 days,
Radioactivity is measured by and instrument Geiger Muller counters.� To enhance the emissions and to calculate accurate emissions certain scintillating liquids are used called scintillation fluids and the counter is called scintillating counter.
1 Becquerel = 1 disintegration per second.
1 Becquerel = 2.7 x 10^-5 uci.
1 uci = 3.7 x 10^4 Becquerel.
1 uci = 2.2 x 10^6 dpm (disintegration per minute) or 3.7 x 10^4 Becquerel
1 Ci = 1000 mci.
1mci = 1000 uci.
Non-radioactive labelling compounds:
As people experienced the hazards of radioactive isotopes to health and difficulty in disposing radioactive waste, they looked for alternative but effective substitutes at the same time as effective as radioactive isotopes.
Many chromogenic and phosphorogenic and luminescent compounds have been identified and some have been synthesized to the needs. Some biological fluorescent components are also in use.
Digoxigenin and Biotin are used for chromogenic purposes.� Digoxigenin is a steroid extracted from Digitalis purpurea and D. elata.� One can obtain antibodies against both digoxigenin and strptavidin, which binds strongly to Biotin (a vitamin).� These can be preserved at -20^oC for more than six months with out any loss of activity. Digoxigenin and Biotin can hooked on to nucleotides such as dUTP or rUTP through specific spacer molecules.� These can be used as any labeled nucleotides for labeling DNA or RNA, which can be used as probes or tracers.� They can be quantified and detected by using chromogenic reactions.� Digoxigenin compounds can be detected by first binding these compounds with specific antibodies, then they are bound by Alkaline Phosphotase conjugated Fc fragment bases anti-antibodies.
�
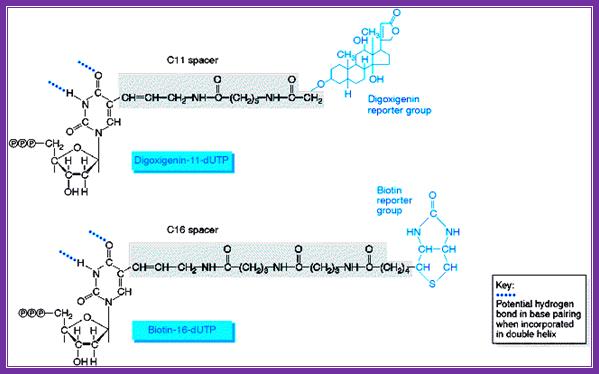
www.markergene.com
In the presence of NBT (Nitro Blue Tetrazolium) and BCIP (5�Bromo 4-Chloro 3- indolyl phosphate), when treated with alkaline Phosphotase yields brown-purplish color, which can be detected.� Biotin labeled compound can be detected first binding with strptavidin, then they can be probed with antibody against strptavidin and then with alkaline Phosphotase conjugated anti-antibodies.
One can also use chemiluminescent protocols, where biotinylated nucleotides can be used for the preparation of probes.� When they are used as the probes when targets are bound to the membrane; streptavidin can be used to bind to biotinylated probe.� Then biotinylated-alkaline phosphate complex is added to bind to strptavidin.� Next a luminogenic compound such as PPD reagent is added.� The AP removes the phosphate group from phenyl-phosphate substituted 1,2 dioxetane (PPD) resulting in a stable intermediate compound which then spontaneously decays emitting light at 447nm.� The emitted light can be caught on X-ray films.
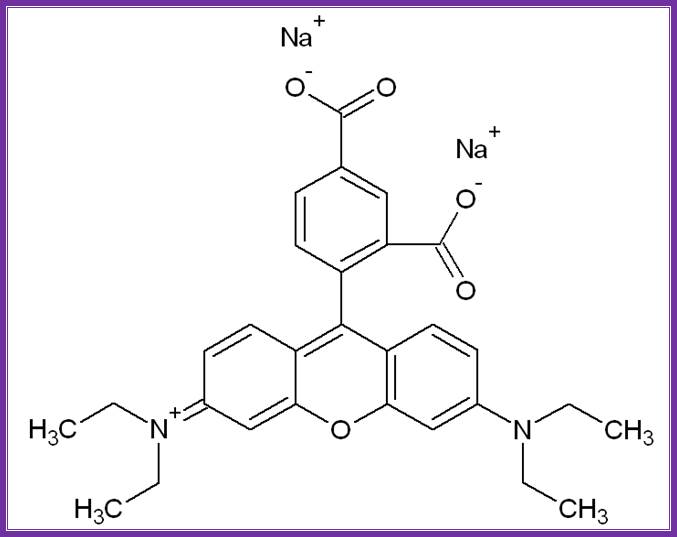
Antibodies against digoxigenin or for that matter any other proteins can be labeled on its Fab fragments by conjugating 5(6)-carboxy-fluoroscien-N-hydroxysuccinimide ester or 5(6)-carboxy-rhodaminr-101-N-hydroxysuccinimide ester.� Such materials can be used to detect DNA fragments on membranes or one can use to detect components at intercellular level or to detect receptors or receptor bound components on the cell surface.� Even these can be used for Chromosomal painting.� They emit light on exposure to UV rays at 260-360nm; Fluorescent emits green color and Rhoda mine emits red color.
Compounds that emit fluorescence or produce color:
|
Name |
Absorption at |
Emission at |
|
Allophycyanin |
610-640 (red) |
660 (red) |
|
Fluorocien isothiocyanate |
450-495nm(blue)` |
520nm (green) |
|
Hoechst 33258 |
360nm (UV) |
470nm (blue) |
|
R-Phycoerythrin |
480-565 (green) |
578 (yellow) |
|
RED-13 |
488nm (green) |
613nm (red) |
|
Rhodamine |
552 |
570nm (red) |
|
Texal red |
596 (green) |
620nm Yellow |
|
Acridine |
|
Orange |
|
Cholesterol |
UV |
Yellow green |
|
Dinitrophenol |
UV |
Yellow |
|
Luciferin (bio) |
+ ATP |
Emit luminescence |
|
B-Galactosidase |
+ X-Gal |
Produce blue color on X-Gal |
|
Gus |
+ Glucuronic acid |
Produces blue color on Glucuronic acid |
|
GFP (green fluorescent protein) 27KD |
|
Exposed UV it emits green color |
�����������������������������

����������������������������� ���������������������������� Texas red;� http//www. en.wikipedia.org
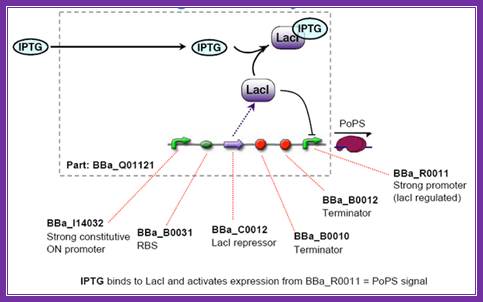
����������������������������� IPTGscreening; http://2006.igem.org/wiki/index.php/ETH2006_iptg

Dinitrophemol; http://www.commons.wikimedia.org

Acridine orange; http;//www.chemistry.about.com
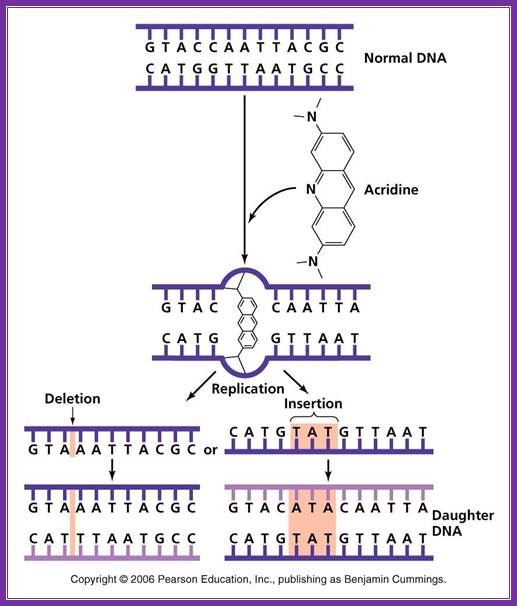
����������������������������������������������������� Acridine: Nonionizing radiation such as ultraviolet light causes the breaking of bonds between purines and pyrimidines when two pyrimidine molecules of the same type (T or C) are adjacent to one another on a nucleoside. These pyrimidine dimers distort the sugar phosphate backbone and prevent proper replication and transcription. �;
������ http://academic.pgcc.edu/~kroberts/Lecture/Chapter%207/mutagens.html
Phycoerythrobilin

Allophycocyanin
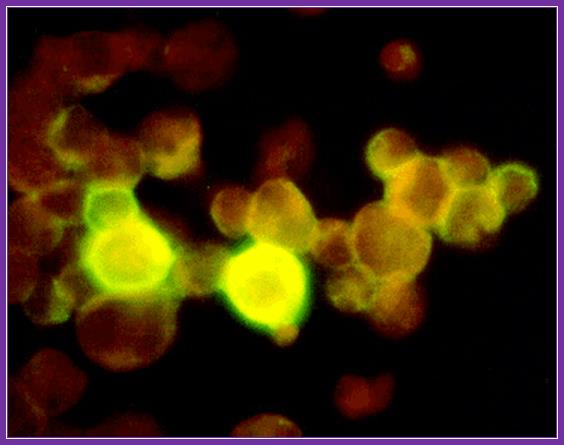
Avidin- flurocien isothiocynate; http://www.ks.uiuc.edu/Research/smd_imd/avidin/
5�bromo-4�chloro isopropyl; http://www.fishersci.com
Digoxigenin; http://lifescience.roche.com/shop/products/dig-high-prime


Fluorescent butterflies; www.appszoom.com
����� Pteridinefluorescence;http://www.chm.bris.ac.uk/motm/folic-acid/folicjm.htm
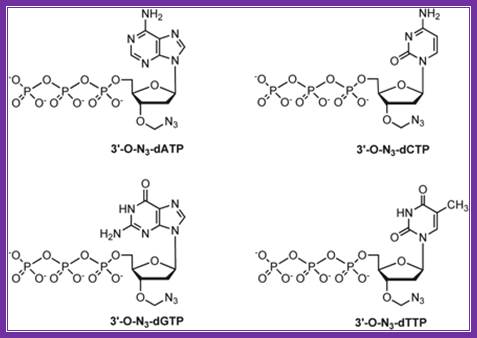
Fluorescent ntds
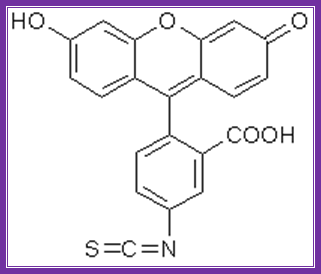
Fluorescien isothiocyante
Labeled nucleotides; http://www.nature.com/
Rhodamine; http://commons.wikimedia.org/wiki/File:Rhodamine_WT.png
Osamu Shimomura and colleagues got Nobel Prize 2008, for their work; Green florescent protein used: the green fluorescent protein luciferase was used in developing fluorescent tobacco plant; www.science.howstuffworks.com; another glowing protein was isolated from florescent jelly fish; Genetics.wikispace.com

Red fluorescent protein used for glowing cats �South Korea; they were the first to clone glowing red-cat; http://news.nationalgeographic.com/