Pre rRNA� Processing in Prokaryotes:
Processing of rRNA, tRNA and mRNA.
Processing of rRNA:
Most of the RNAs transcribed are precursor RNAs and they have to be processed to their functional state.� Ribosomal RNAs are synthesized as large precursors and as they are synthesized, they are subjected to processing, which involves, methylation (modifications) and pseudouridylation of bases at specific sites by using secondary structural conformations; then cleaving the precursor transcript at specific positions. Methylases, methylate 4-O-guanine bases (GC pairs) and 2-O- group of ribose sugar.� Methylation is very important in the binding of riboproteins to rRNA and also for the folding of rRNA as and when proteins bind to form a ribosomal structure.
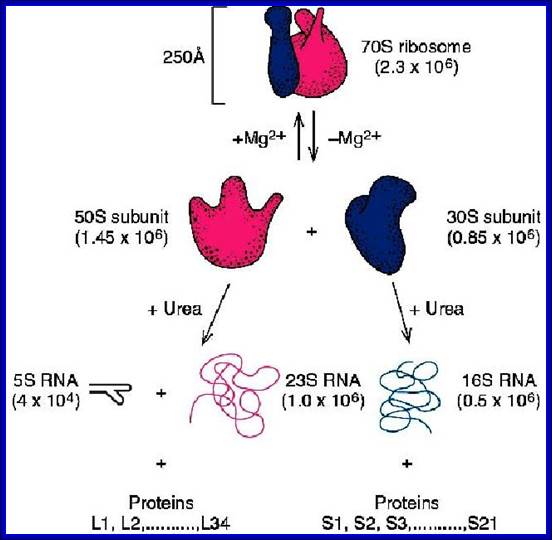
Weaver, Molecular Biology, 2E; http://biology.kenyon.edu/
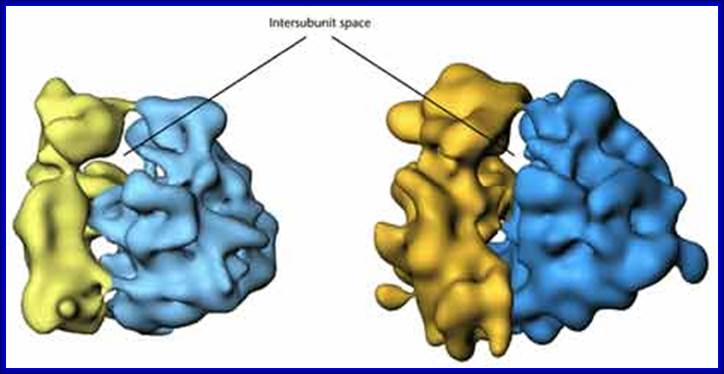
Eukaryotic Ribosomal subunits; https://www.cancerwatch.org
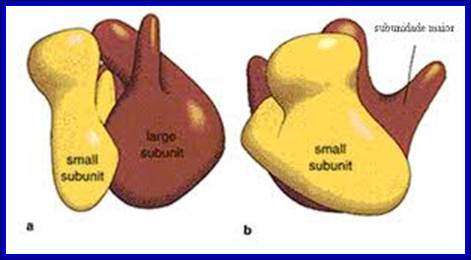
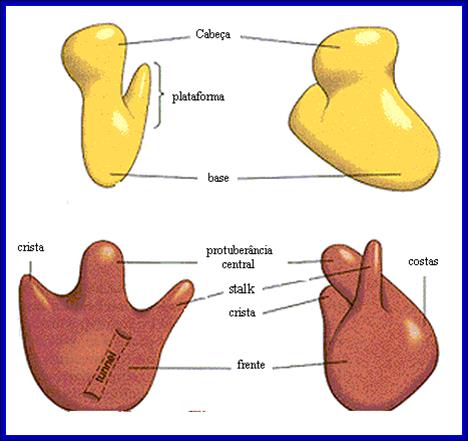
http://www.icb.ufmg.br/
http://www.icb.ufmg.br/
|
Various Kinds of RNA Found in an E. coli Cell |
|
||||||
|
Type |
Sedimentation |
Molecular Weight |
Number of |
Percentage of |
|||
|
mRNA |
6�25 |
25,000�1,000,000 |
75�1000 |
~2 |
|||
|
tRNA |
~4 |
23,000�30,000 |
73�94 |
16 |
|||
|
rRNA |
5 |
35,000 |
120 |
82 |
|||
|
Non coding RNA (NOCORNAc) |
Ex. RNA-6sRNA |
|
38 intergenic transcripts |
||||
|
|
|
|
|
||||
Number of rRNA and tRNA Genes:
|
Species |
16s/23s/5s or 18s/28s-5.8s� rRNA genes |
5s rRNA genes |
tRNA genes |
|
E.coli |
7 |
Embedded in rRNA end segment |
~60 |
|
S.cerviciae |
140 |
140 |
250-400 |
|
Dictyostelium discoideum |
180 |
180 |
>850 |
|
D.melanogaster: ������������������������ XY: ����� �������������������XX: |
150 250 |
150 150 |
850 850 |
|
X.laevis |
450-600 |
24000 |
1150 |
|
Homo sapiens |
280 |
2000 |
>2000 |
|
|
|
|
|
Size of Precursor and Processed rRNAs:
|
Species |
Precursor |
Pre 23s/28s in ntds |
16s/18s final in ntds |
Percentage �Of precursor |
|
E.coli |
5.6 to 6 kb |
2914-3100 |
1500 |
80% |
|
Yeast37s |
7.2-8.95 kb |
3750-3800 |
1700-2000 |
80% |
|
Dictyostelium |
7.4 kb |
4100 |
1800-2000 |
|
|
Drosophila |
7-7.4 kb |
4100 |
1800 |
78% |
|
Xenopus |
7.875 kb |
4475-4500 |
1900-1925 |
79% |
|
Gallus domesticus |
11.25 kb |
4625 |
1800 |
57% |
|
Mus musculus |
12.4-13kb |
4712-5100 |
1900-1958 |
52% |
|
Homo sapiens |
13.7kb |
5100 |
1900 |
|
|
Plants (general) |
7.9kb |
3700 |
1700 |
71% |
|
Types |
RNA size |
Number of ribo proteins |
Methylations |
|
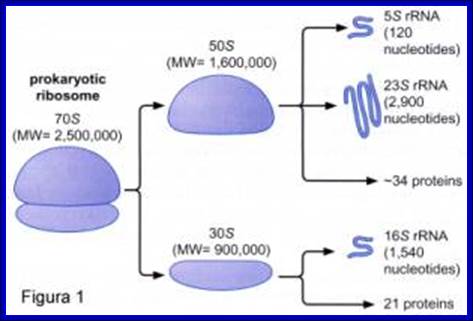
70S ribosomal RNAs. (20nm)
|
�Coded by seven RNA operons and 6 protein operons |
|
|
|
30s subunits |
16s RNA, 1540-42 ntds |
21 (s1 to s21) |
Ten, 2�-OH, methyl adenines, 2�-OH, dimethyl guanines, �help in folding and processing. |
|
50S subunits |
23s RNA, 2900 ntds; 5s RNA, 120 ntds |
34, L1 to L34 |
Twenty,2-OH methylations Total 10 psuedouridines |
|
80S ribosomes: 25-35nm |
Coded by hundreds of genes |
|
|
|
40S subunits |
18s RNA;( 1843 Or 1900 ntds) |
33; S1 to s34 |
43 to 44 methylations at 2�OH groups, plus conversion of certain Uridine into pseudo-Uridines |
|
60s subunits |
28s-RNA;(4718- 4800 ntds); 5.8s RNA;(160ntds); 5s RNA;(120ntds); Note; 45S rRNA genes are located in nucleolar region of chromosomes 13,14,15,20 and 21 |
49; L1 to L45-50 |
74 methylations at 2�OH of sugars, Methylation at adenine, Methylation at guanine, plus conversion of Uridine into pseudo-Uridines. The number of Uridinylation and methylation sites vary |
|
Mitochondrial ribosomes: Mammalian-55-56s
Maize- Maize-78s,
Potato 33s/50s, |
30s=12s RNA 50s=16s RNA 12s=954nts, 16s=1558nts No 5s. in Hu it is imported
26s,18.5s,5s
26s,18s, 5s |
28s-1560 ntds, 48 proteins -12s -954 nts,29 proteins
|
NO 5s in mammals, but imported
|
|
Chloroplast ribosomes: 70s |
50s and 30s |
23sRNA, 16s RNA 5s RNA, 4.5s RNA |
|
|
C. reinhardtii, 70s |
50s + 33s , |
|
|
|
Pea Plastid,70s -
|
50s and 30s |
23s 16s 5s |
|
|
|
|
|
|
Human- rRNA gene are located on chromosomes 13, 14, 15, 21 and 22 (Nor region), located at 13p12, 14p12, 15p12, 21p12, and 22p12; 5s rRNA genes~ 100 copies, located on chromosome 1-1q42.
Drosophila- rRNA genes located on X -496 genes and Y chromosomes-1445 genes, 5s 100 copies,
Yeast- rRNA genes located on chromosome XII, 150-200 copies.
E.coli genome in condensed state and sites of transcription:
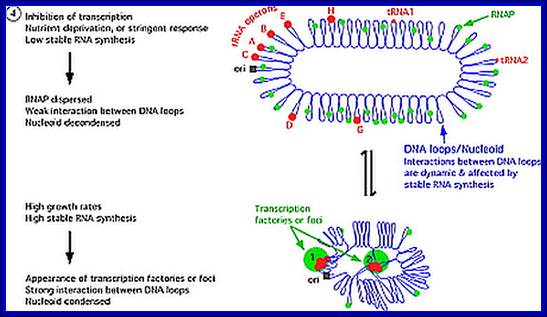
E.coli genome is condensed state with many loops, specific loops containing ceretain gens are engaged in transcriptional activity, transcriptional loci or called transcriptional factories are shown in green. Model linking stable RNA synthesis, RNAP distribution and the dynamic structure of the nucleoid: The E. coli chromosome is represented as blue lines folded in loops, the ori of replication as a black square, the seven rRNA operons as large red circles with letters, and two representative tRNA operons as small red circles. The RNAP molecules are represented as small green circles. For simplicity, only two putative transcription factories/foci, which make the nucleoid more compact by pulling different stable RNA operons into proximity, are indicated here (bottom part of the diagram, large green circles labeled 1 and 2) [adapted from the study by �(Cabrera and Jin, 2003). There are seven rRNA� operons and six ribosomal protein operons.
rRNA operons:
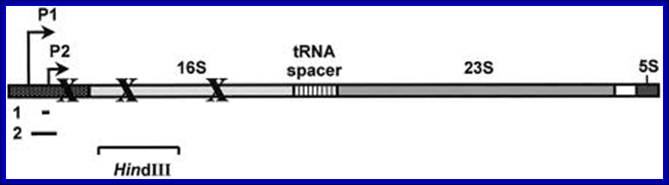
In E.coli Ribosomal RNA genes are organized into seven operons, called rrns and ribosomal protein genes into six operons.� Ribose RNA operons, seven of them are dispersed in bacterial chromosomes.� These operons have two promoters, P1 and P2, separated by about 100bps. E.coli rRNA operons are transcribed by two tandem promoters, rrn P1 and rrn P2, with P1 being the predominant promoter at medium to fast growth rates.
All seven P1 core promoters contain the consensus -10 hexamer (TATAAT) and close matches to the consensus -35 hexamer (TTGACA). �The direction of transcription is same as that replication. The operons are rrn A, rrn B, rrn C, rrn D, rrn E, rrnG, rrn H, in some rrnF is present in place of rrn-H. Transcripts are larger than the final products.� Each precursor RNAs contains non-coding spacer and also tRNA segments are found in spacers.
Typical rrn operon of E.coli; http://www.jbc.org/
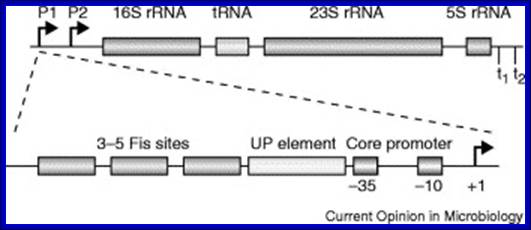
Schematic organization of one of the seven rRNA operon in Escherichia coli: The primary transcript contains all three rRNA species (16S, 23S and 5S), as well as one or more tRNAs transcribed from two promoters, rrn P1 and rrn P2. The transcript is terminated by two terminators, t1 and t2. The rrn P1 promoters consist of three to five binding sites for the transcription activator Fis, an UP element (the binding site for the α subunits of RNAP), and a core promoter element (containing the −10 and −35 hexamers for binding the σ subunit of RNAP). http://www.bx.psu.edu
E.coli rRNA operons are transcribed by two tandem promoters, rrn P1 and rrn P2, with P1 (in rrnB) being the predominant promoter at medium to fast growth rates.� All RNA�s, are transcribed by the same RNAP using sigma 70. All seven P1 core promoters contain exact matches to the consensus -10 hexamer (TATAAT) and close matches to the consensus -35 hexamer (TTGACA).
P1 promoter --- (consensus)-- -35 hexamer (TTGACA--(-)10- hexamer (TATAAT)
The operons are rrn A, rrn B, rrn C, rrn D, rrn E, rrnG, rrn H, in some rrnF is present in place of rrn-H. Transcripts are larger than the final products.� Each precursor RNAs contains non-coding spacer and also tRNA segments in spacers.
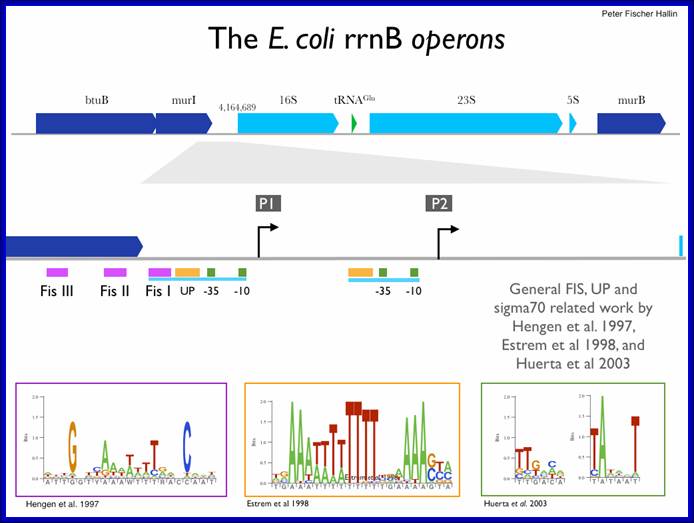
Fis = Factor Inversion binding Site. http://www.cbs.dtu.dk/
Among the two promoters P1 and P2 of rrnE operon lower concentration of ntds uses P2 otherwise the P1 is used; the start point for P1 is ATP (GTP) and for P2 it is CTP. Fis 98a.a dimer (Factor for inversion and stimulation) binds upstream of rrnP promoters and interacts with RNAP and induces DNA binding. Promoters containing Fis binding site activates rrnB promoters 20-30%; http://www.cbs.dtu.dk/
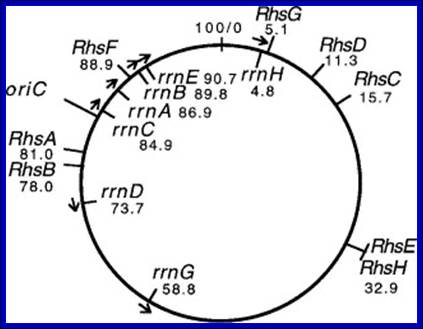
E.coli genetic map with positions of seven rRNA operons
E.coli, contains 56 tRNA genes. A cluster of nine tRNA genes located in the 1-kb region between ribosomal operons rrnJ and rrnW in Bacillus subtilis has been cloned and sequenced. This cluster contains the genes for tRNA(UACVal), tRNA(UGUThr), tRNA(UUULys), tRNA(UAGLeu). tRNA(GCCGly), tRNA(UAALeu), tRNA(ACGArg), tRNA(UGGPro), and tRNA(UGCAla). The newly discovered tRNA gene cluster combines features of the 3'-end of trnI, a cluster of 6 tRNA genes between ribosomal operons rrnl and rrnH, and of the 5'-end of trnB, a cluster of 21 tRNA genes found immediately 3' to rrnB. Neither the tRNAu'5ZG gene nor its product has been found previously in B. subtilis. In B. subtilis tRNAs are found clustered between rRNA operons. In B. subtilis at the 3� end of rRNA genes one finds a cluster of 21 tRNAs.
16s-23s-5s----6tRNAs (trnl)-16s-23s-5s-16s-23s-5s,
16s-23s-5s----9tRNAs (trnJ)-16s-23s-5s-
Organization of tRNA and rRNA [Rrn]-Operons in Bacteria:
|
Operon |
16s |
Spacer tRNA |
23s |
5s |
Spacer tRNA |
Mpu site |
|
Rrn-A |
+ |
Ile, ala |
+ |
+ |
- |
86 |
|
Rrn-B |
+ |
Glu (2) |
+ |
+ |
- |
89 |
|
Rrn-C |
+ |
Glu |
+ |
+ |
Asp, trp |
84 |
|
Rrn-D |
+ |
Ile (2), ala (18), |
+ |
+ |
Thr |
70 |
|
Rrn-E |
+ |
Glu (2) |
+ |
+ |
- |
90 |
|
Rrn-G |
+ |
Glu (2) |
+ |
+ |
- |
56 |
|
Rrn-H (F) |
+ |
Ile, ala (18), |
+ |
+ |
Asp |
5 |
|
tRNA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Some of the tRNAs are organized into polycistronic operons as shown below, eg B. subtilis, otherwise they are located in the middle of 16s and 23s rRNA segments and many at 3� end beyond 5s segment. A cluster of 21 tRNA genes found immediately 3' to rrnB (fig. shown below.

B subtilis, rrnB/trnB operon, P and T promoter and terminator respectively;
E.coli Operon with its 16s, 23s and 5s coding region often contain tRNA also. These are then processed by specific enzymes.
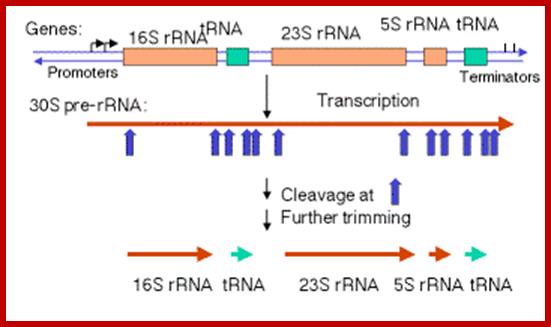
The Maturation of rRNA in E, coli.
The primary transcript of the rrnB operon is used here as a schematic model. The other six rRNA-encoding operons are similar in principle but might differ in the number and identity of the tRNAs encoded by the intergenic spacer for example, rrnB encodes glt; rrnA encodes ileT and alaT), example, rrnB encodes gltT; rrnA encodes ileT and alaT), the number of 5S rRNA-encoding sequences (rrnD encodes two, along with four tRNAs) and the number of Rho-independent terminators.
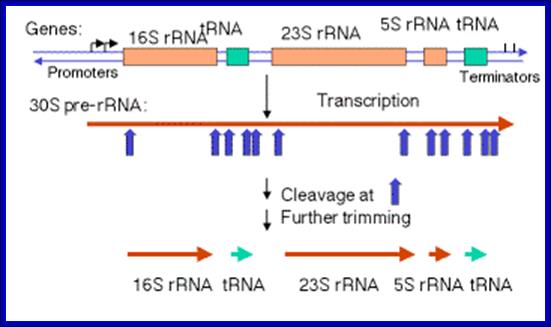
Precursor rRNA processing sites; George A. Mackie http://www.nature.com/
The precise order of events is not known, but it seems likely that folding and modification of the initial transcript, and the addition of ribosomal proteins occurs before the action of RNase III. Sequences in the leader and spacers anneal to form the processing stems, which are susceptible to the action of RNase III52, 69. RNase III makes staggered cleavages to release pre-16S (17S), pre-23S and pre-5S rRNAs.
These in turn bind the remaining ribosomal proteins, undergo modifications and are cleaved by combinations of RNase E, RNase G, RNase T and unknown enzymes (at the 3′ end of the 17S rRNA and the 5′ ends of the pre-23S and pre-5S rRNAs). For simplicity, helicases and modifying enzymes are not shown, although they are important for these processes. The maturation of tRNAs involves RNase P and a combination of 3′ exonuclease, including RNase PH, RNase T, RNase D, RNase BN, RNase II and polynucleotide phosphorylase (PNPase). George A. Mackie http://www.nature.com/
(1)The segment containing 16S rRNA (small ribosomal subunit) and the one containing 23S rRNA (large ribosomal subunit) are flanked by inverted repeats that form stem structure in the RNA.
�(2)The stems are cleaved by RNase III. There is no apparent single sequence at which RNase III cleaves ‑ perhaps it recognizes a particular stem structure. This plus subsequent cleavage events (by an activity called M16) generates the mature 16S and 23S rRNAs. The rRNAs are also methylated.
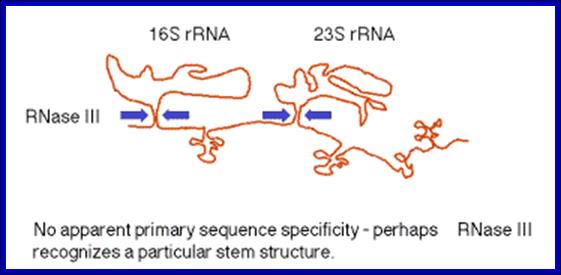
RNase III cuts in the stems of stem-loops in RNA; RNA is liberated by RNases P and F. 5S rRNA is liberated by RNases E and M5; http://biowiki.ucdavis.edu/
Excision of rRNAs and tRNAs from 30S precursor RNA; http://biowiki.ucdavis.edu/
The ends of tRNA in E. coli are produced by the action of three nucleases that cleave the precursor to tRNA. A schematic of the pre-tRNA is shown at the top, with RNA extending from the 5� and 3� ends of the RNA that will become the mature tRNA (shown as a cloverleaf). The site of cleavage is indicated by the short vertical arrows above the lines denoting RNA, and they are labeled with the name of the enzyme cutting at that site. The enzymes catalyzing each reaction are listed above or adjacent to the reaction arrows. http://biowiki.ucdavis.edu/
Pre tRNA-
RNasess P, F, D cut in sequence specific manner. RNase P cleaves at 5� end of the tRNA, RNase F cleaves at 3� end and RNase D trim from 3� direction to the 3� end of the tRNA.
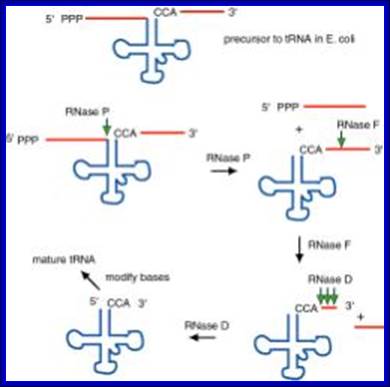
The ends of tRNA in E. coli are produced by the action of three nucleases that cleave the precursor to tRNA. A schematic of the pre-tRNA is shown at the top, with RNA extending from the 5� and 3� ends of the RNA that will become the mature tRNA (shown as a cloverleaf). The site of cleavage is indicated by the short vertical arrows above the lines denoting RNA, and they are labeled with the name of the enzyme cutting at that site. The enzymes catalyzing each reaction are listed above or adjacent to the reaction arrows.
RNaseP- The catalytic RNaseP is 375nts long and it is associated with 20kDa protein, Catalytic activity is performed by RNA itself and the protein assists in cleavage reaction. This was one of the first instances discovered of catalytic RNA, and Sidney Altman shared the Nobel Prize for this.
Ribosomal RNA and tRNA processing and modifying enzymes:
16s Methyl transferase-sun protein-2 copies,
16s rim M,
16S Pseudo Uridine rluA,
16S pseudouridine 516 synthase rsuA,
23S pseudouridine synthase C rluC� 2copies,
23S modification pseudouridine synthase D rluD,
Dimethyl adenosine transferase- KsgA,
S adenocyl methioninet RNAribosyl transferase-isomerase-queA,
tRNA methyl transferse 95-methylaminomethyl-2-thiouridylate) trmU,
tRA guninyl N1-methyl transferase trmD
tRNA guanosine2�omethyl transferasetrnH.
tRNA uacil-5-methyl transferase trnA,
tRNA delta(2)-isopentenyl pyrophosphotransferase miaA,
tRNA pseudouridine synthase truA
tRNA pseudouridine synthase yru B
Ribosomal Protein Operon (RP): S10 consists of 11 protein coding cistrons
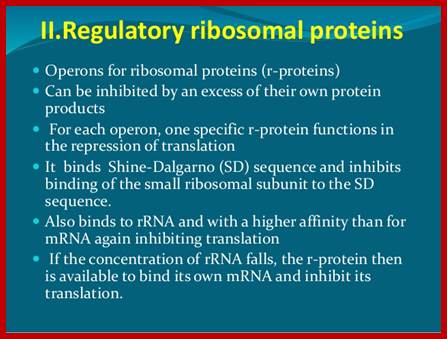
https://www.slideshare.net
Ribosomal Protein coding operons:
L11:� [--] ---L11�L1. [L1 blocks at L11].
Beta: [--]�L10�L7/L12--b--b�.[L10 blocks at L10].
Str:�� [--]�s12�s7�EF-G�EF-Tu. [S7 blocks at S7].
Alpha: [--]�s1---3�s11�s4---a---L17. [S4 blocks at S13].
S10:� [--]�s10�L3�L4�L23�L2�L19--L22�s3�L16�L29�s17. [L4 blocks at S10]
Spc: [--]�L14�L24�L5�s14�s8�L6�L18�s5�L30�L15. [s8 blocks at S10]
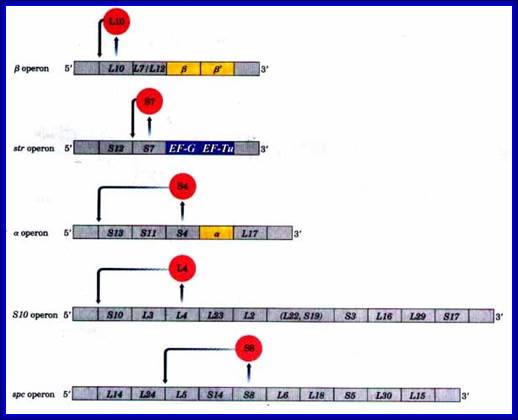
http://www.bioinfo.org.cn
tRNA genes Embedded in rRNA operons :
|
Operons |
tRNA genes encoded in rRNA operon |
|
rrnA |
tRNA-Ile, tRNA-Ala |
|
rrnB |
tRNA-Glu |
|
rrnC |
tRNA-Glu, tRNA-Asp, tRNA-Trp |
|
rrnD |
tRNA-Ala, tRNA-Ile, tRNA-Thr |
|
rrnE |
tRNA-Glu |
|
rrnG |
tRNA-Glu |
|
rrnH |
tRNA-Ala, tRNA-Ile, tRNA-Asp |
|
tRNA Operon- Polycistronic |
valV valW and leuQ leuP leuV |
|
tRNA Operon-Plycistronic |
glyW cysT leuZ and argX hisR leuT proM |
|
tRNA Operon-Plycistronic |
2RNAmMet,2RNA1Gln 2tRNA2Gln,tRNAX |
Bacteria synthesize three different rRNAs, called 5S rRNA, 16S rRNA and 23S rRNA, indicating the size of the molecules as measured by sedimentation analysis (see Technical Note 2.2). The three genes for these rRNAs are linked into a single transcription unit (which is usually present in multiple copies, seven for E. coli) and so the pre-rRNA contains copies of all three rRNAs. Cutting events are therefore needed to release the mature rRNAs. These cuts are made by various ribonucleases, at positions specified by double-stranded regions formed by base-pairing between different parts of the pre-rRNA. The cut ends are subsequently trimmed by exonucleases.
|
Table: Comparison of Ribosomal RNA Modifications: Species from Three Phylogenetic Domains; (E) = Eukaryote, (B) = Bacterium, (A) = Archaeon. Data from [Bachellerie & Cavaille, 1998,Ofengand & Fournier, 1998,Noon et al., 1998]. |
||||||||||||||||||||||||||||||||||||
|
Pseudouridine
rRNA modifications (![]() ) are numerous in eukaryotes and few in bacteria and archaea. Studies
of eukaryotic
) are numerous in eukaryotes and few in bacteria and archaea. Studies
of eukaryotic ![]() residues show they are found in the most evolutionarily conserved
regions of rRNA.
residues show they are found in the most evolutionarily conserved
regions of rRNA. ![]() are spread throughout SSU rRNA with no clear association with
particular functional regions. In contrast, to LSU rRNA
are spread throughout SSU rRNA with no clear association with
particular functional regions. In contrast, to LSU rRNA ![]() residues are clustered in three main regions, all within or
structurally associated with the Peptidyl transfer center (PTC) of the
ribosome. Individual loss of
residues are clustered in three main regions, all within or
structurally associated with the Peptidyl transfer center (PTC) of the
ribosome. Individual loss of ![]() residues is not lethal [Ni et al., 1997,Gannot et
al., 1997], although global loss of pseudouridylation due to
mutations in the putative pseudouridine synthase, Cbf5p, causes
temperature-sensitive growth impairment. It is thought
residues is not lethal [Ni et al., 1997,Gannot et
al., 1997], although global loss of pseudouridylation due to
mutations in the putative pseudouridine synthase, Cbf5p, causes
temperature-sensitive growth impairment. It is thought ![]() residues play a variety of roles in the ribosome, some improving
translational efficiency, others with undetermined function.
residues play a variety of roles in the ribosome, some improving
translational efficiency, others with undetermined function.
Methylation of RNA occurs at a variety of atoms, nucleotides, sequences and tertiary structures. Strongly related to other posttranscriptional modifications, methylation of different RNA species includes prokaryotic and eukaryotic tRNA, rRNA, mRNA, tmRNA, snRNA, snoRNA, miRNA, and viral RNA. Different catalytic strategies are employed for RNA methylation by a variety of RNA-methyltransferases which fall into four super families. This just outlines the different functions of methyl groups in RNA, including biophysical, biochemical and metabolic stabilization of RNA, quality control, resistance to antibiotics, mRNA reading frame maintenance, deciphering of normal and altered genetic code, selenocysteine incorporation, tRNA aminoacylation, ribotoxins, splicing, intracellular trafficking, immune response, and others. Connections to other fields including gene regulation, DNA repair, stress response, and possibly histone acetylation and exocytosis are pointed out. WIREs RNA 2011 2 611�631 DOI: 10.1002/wrna.79.
Processing means site specific modification at ribose OH �sites and site-specific bases by 16s rRNA methylases, this provides resistance to aminoglycosides. N6-methyladenosine, N6-dimethyladenosine, 5-methylcytidine (m5C), 3-methyluridine, and N2-methylguanosine were found.� The 50s 23s rRNA is also methylated at G645 at N1 position. The E. coli 16S and 23S rRNAs contain 11 and 23 modifications respectively, and in many cases the enzymes responsible for these modifications are known. However, Pseudouridinylation of rRNA is known, but the mechanism is not clear. Such modifications lead to folding and cutting of rRNA precursors and then bind to respective riboproteins and organize into functional ribosomal subunits.
RNase-P:
- RNase-P is a unique enzyme, for it is made up of 375-377 ntds long RNA associated with protein subunits of 20kd and 14 KD. This type of RNA-by itself functioning as an enzyme is found in both prokaryotes and eukaryotes.�� The RNA part has certain secondary structure through which it recognizes the tRNA domain and associates and cleaves to produce the 5� of the tRNA, whether the tRNA is found in spacer regions of ribosomal RNA precursor or it can be tRNA precursor transcript.
�
- The amazing property of this complex is, the RNA part itself can perform cutting in site-specific manner, which is an endonucleolytic cleavage.� The only cofactor the RNA requires for the process is little higher concentration of Mg^2+; its efficiency increases.�
Because of the said features, this P- complex is called Ribozyme. Such Ribozymes are found in eukaryotes and cell organelles; ex. Mitochondria contains ribozyme called MRNP.
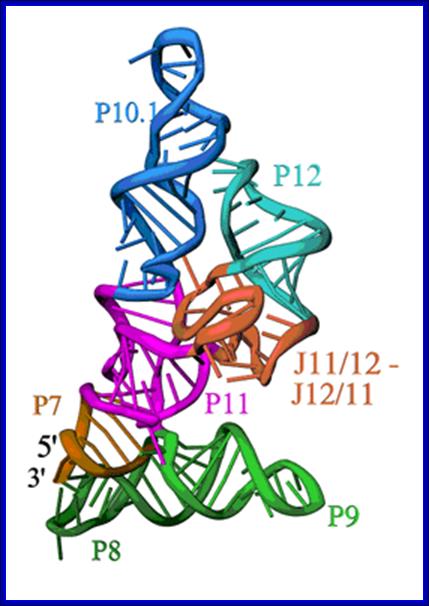
Secondary structure of RNA component of RNase-P; �Structure of the specificity Domain of Bacterial RNase P; Ribbon diagram of the S-domain of B. subtilis RNase P. The P7-P11 stems form a cruciform, with one helix in the cruciform formed by the stacking of P8 and P9, and the other by the stacking of P7, P10 and P11. The GAAA tetraloop at the end of P12 interacts with the tetraloop receptor in P10.1. The highly conserved J12/11-J11/12 module forms a large structure devoid of Watson-Crick base pairs. Andrey S. Krasilnikov et al; https://www-ssrl.slac.stanford.edu
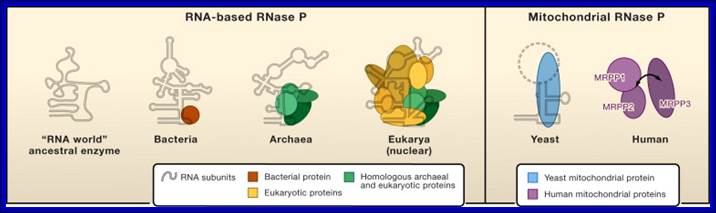
Such RNase-Ps are also found in other bacteria, some contain C5 protein with the RNA, archaea contain 4-5 proteins, eukaryotes too contain RNaseP with 9-10 proteins.� Mitochondrial RNase P is just protein no RNA.� Human mitochondrial RNaseP and plastid RNaseP (70kd) contain only protein but no RNA. Mitochondrial ribozyme is called MRNP
The Evolution of RNase P: The compositions of characterized RNA-based RNase P enzymes from bacteria, archaea, and eukarya show an increase in protein content with increased complexity of the organism. The sites of interaction between RNase P subunits are not known in most cases and are represented schematically. The structure of the proposed ancestral RNA-only RNase P is not known and is assumed to have the critical structural elements conserved in all forms of RNase P RNA. The composition of the fully characterized mitochondrial RNase P is shown for yeast (S. cerevisiae) and human (H. sapiens). Human mt RNase P is composed only of proteins (mitochondrial RNase P proteins 1, 2, 3) (Holzmann et al., 2008). The third subunit of the human mt RNase P (MRPP3) binds to the two-protein sub complex weakly and may associate dynamically (arrow).
Although key structural elements of the RNA subunit are preserved in various yeast mtRNase P enzymes (solid line), the entire RNA structure is not well defined (dashed line). The Evolution of RNase P(Left). The compositions of characterized RNA-based RNase P enzymes from bacteria, archaea, and eukarya show an increase in protein content with increased complexity of the organism. The sites of interaction between RNase P subunits are not known in most cases and are represented schematically. The structure of the proposed ancestral RNA-only RNase P is not known and is assumed to have the critical structural elements conserved in all forms of RNase P RNA.(Right) The composition of the fully characterized mitochondrial RNase P is shown for yeast (S. cerevisiae) and human (H. sapiens). Human mtRNase P is composed only of proteins (mitochondrial RNase P proteins 1, 2, 3). The third subunit of the human mtRNase P (MRPP3) binds to the two-protein subcomplex weakly and may associate dynamically (arrow). Although key structural elements of the RNA subunit are preserved in various yeast mtRNase P enzymes (solid line), the entire RNA structure is not well defined (dashed line). (Holzmann et al., 2008).
- It is not the only RNA found in RNase-P complex (first one to be discovered) that is capable of performing enzymatic activities; it is now known there are several such RNAs, which contain certain domains capable cleaving RNA at specific position.
Processing of tRNAs found within rRNA Operons:
The precursor tRNA, as they are synthesized, they fold into secondary structures, possibly assume a 3-D form.
�
- RNase-P recognizes the sec. structure, binds to it and cleaves exactly at 5� end of the tRNA.
�
- At the other end RNase-D and endonucleases (first RNase E/Fs from 3�end) recognizes 3� region and cuts , then RNaseD chops off nucleotide by nucleotide from 3� end till it reaches secondary structure with a --CCA 3� sequence and stops, thus processed tRNAs are released.
- The bases of tRNAs are then modified by a variety of enzymes. Modification of bases by specific enzymes, at specific sites is performed. The modifications are methylation, hydroxylation, thiolation, amination, deamination and few others.
- In some the tRNA liberated lacks CCA sequence at 3� end.� In such cases these nucleotides are added in sequence by tRNA terminal transferase.
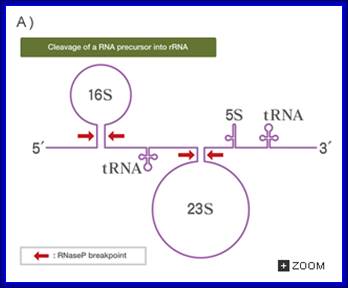
Post transcriptional Modifications; cleavage of precursor rRNA into rRNA and tRNA; Web text book;
The university of Tokyo; http://csls-text3.c.u-tokyo.ac.jp/
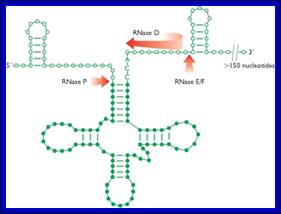
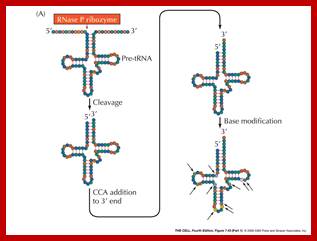
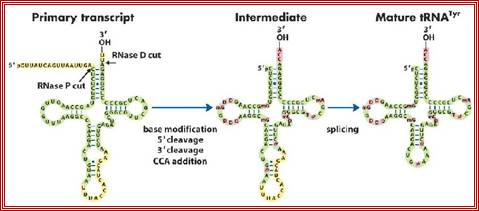
In both bacteria and eukaryotes, tRNA genes occur singly or as multi-gene transcription units.� In bacteria, they are embedded as infiltrators within the rRNA transcription unit. The pre-tRNAs are also processed by a series of ribonucleases, as illustrated in the figure above.� All mature tRNAs must end with the trinucleotide 5′-CCA-3′. Some tRNAs have this sequence already; those that do not, or from which the 5′-CCA-3′ has been removed by the processing ribonucleases, are added by tRNA nucleotidyl transferase.
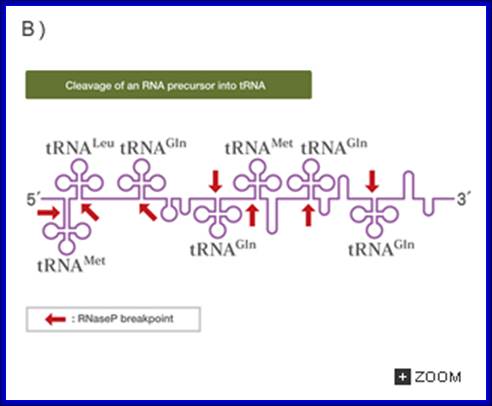
CSLS-Univ. of Tokyo; http://csls-text3.c.u-tokyo.ac.jp/
Processing of Bacterial Transcripts with introns:
Consistent with the concept that introns can function as transposable elements, and those nuclear introns derived from self-splicing group II introns, which then evolved in partnership with the spliceosome. These introns are among the select group of catalytic RNAs that have helped spark interest in the role of RNA catalysis in current cellular processes, as well as in the possibility of a primordial �RNA world�. The splicing mechanism used by group II introns is similar to that of nuclear spliceosomal introns, which has led to suggestions that the former are the progenitors of the latter. Group II introns have also been linked to retro transposable elements, because some of them have been found to encode a functional reverse transcriptase.
It is widely believed that the ribozyme (catalytic RNA) core of group II introns, or some evolutionarily related molecule, gave rise to the RNA components of the spliceosomal splicing machinery of the eukaryotic nucleus. Group II introns seemed to be confined to mitochondrial and chloroplast genomes. The discovery of group II introns both in cyanobacteria (the ancestors of chloroplasts7) and other bacteria I now reported.
Group I introns:
Group I introns are widely distributed in protists, bacteria and bacteriophages. Nearly 1500 group I introns found thus far in nature (e.g. in algae and fungi) has only recently been clarified.
Group I introns are common in the 23s rRNA genes of mitochondria and chloroplasts. Often, they encode �homing endonucleases,� which target highly conserved gene sequences and drive inter-organellar intron mobility, even across species and genus lines. Most bacterial 23S rRNA genes show some of these endonuclease-sensitive target sequences. However, only two bacterial 23S rRNA genes are known to contain group I introns. Until such introns are sliced out the rRNAs cannot function.
Bacterial mRNAs, unlike eukaryotic mRNA, are polycistronic with spacers in between. The 5�UTR and 3�UTR sequences have role in the stability of mRNAs. �Introns have been found in highly expressed genes in eubacteria, bacteriophages, mitochondria and chloroplasts. The said introns are like eukaryotic Group I and Group II category.� Until they are spliced out mRNAs cannot be translated. Clearly, there must be some means of balancing splicing of bacterial introns with co-transcriptional translation.
Semrad and Schroeder (1998) provided the surprising answer that splicing of a group I intron from phage T4 is facilitated by translation of the upstream open reading frame (ORF). This enhancement of splicing is achieved by modulating the long-range conformation of the pre-mRNA. Their results provide useful analogies for the coupling of eukaryotic pre-mRNA splicing with transcription. The introns are found in genes encoding thymidylate synthase (td), ribonucleotide reductase (nrdB) (Belfort 1990), and anaerobic ribonucleotide reductase (sunY, or nrdD) (Young et al. 1994). Self-splicing requires that the intron RNA fold into a unique secondary and tertiary structure (Cech and Herschlag 1996).
����������������������������������������������� Semrad and Schroeder 1998
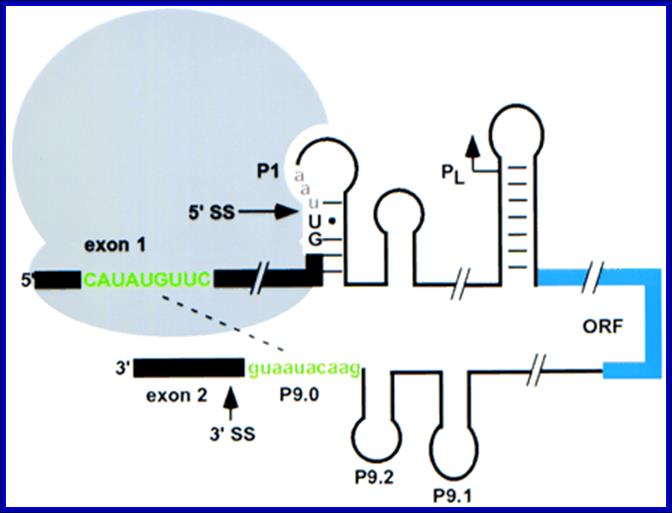
Translation enhances splicing of the td group 1 intron from phage T4 in vivo. Recognition of the 3′ splice site (3′ SS) is inhibited by base-pairing of the 5′ exon with the 3′ end of the intron (green); this pairing is prevented by ribosomes bound upstream (Semrad and Schroeder 1998). Ribosomes may also stabilize the folded structure of the intron. Translation of the pre-mRNA terminates at a stop codon (red) after the 5′ splice site (5′ SS). The internal double-strand DNA endonuclease I-Tev I is indicated in blue; the core of the td intron is omitted for clarity. Adapted from Belfort (1990). In bacteria splicing is coordinated with translation.; it is self-splicing. Contain group I and II introns. Translation requires mRNA to be free from sec structure.
Splicing of a group I intron from phage T4 is facilitated by translation of the upstream open reading frame (ORF), modulates secondary structure in such a way the splice sites are brought closer so the self-splicing takes place.� Small hairpins can form in 10�100 μsec, and tRNAs can fold within milliseconds.
Clostridium difficile: c-di-GMP binding by the Riboswitch induces folding changes at atypical splice site junctions to modulate alternative RNA processing. Some group I introns encode homing endonuclease (HEG), which catalyzes intron mobility. It is proposed that HEGs move the intron from one location to another, from one organism to another and thus account for the wide spreading of the selfish group I introns.
Group I and group II introns are not only catalytic RNAs, but also mobile genetic elements. The success of these introns as mobile elements almost certainly relates to their innate self-splicing capability, which enables them to propagate by inserting into host genes while only minimally impairing gene expression. Nevertheless, both types of introns have become dependent on proteins for efficient splicing in vivo to help fold the intron RNA into the catalytically active structure. Base pairing 1-`10 helices-Introns in cyanobacterial mRNAs is a requirement for self-splicing.� Such introns are found in 23s bacterial rRNA- thermophilic bacteria Thermotoga. Group I intron in the recA gene of Bacillus anthracis, require GTP.
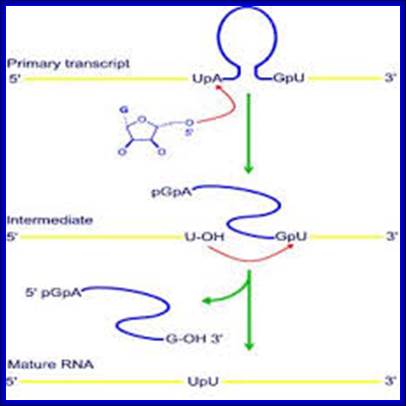
http://nitro.biosci.arizona.edu/
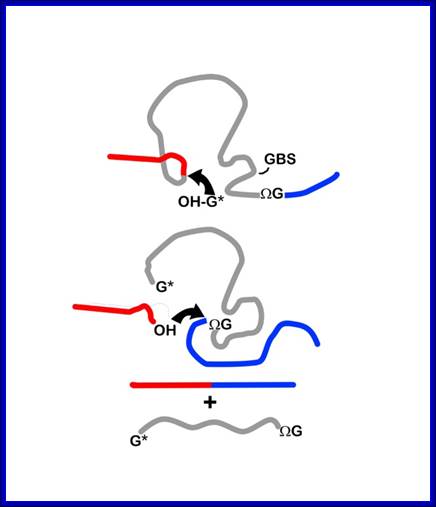
Mechanism of group I intron splicing. 5' and 3' exons are in red and blue, respectively. ΩG, terminal intron guanine. G*, exogenous guanosine. (Step I) Nucleophilic attack on the 5' splice site by the 3'-OH of G* in guanosine-binding site (GBS). (Step 2) Nucleophilic attack on the 3' splice site by the free 3'-OH of the 5' exon. (Step 3) Free intron and spliced exons. (Rahul Raghavan and Michael F. Minnick (2009). Group I Introns and Inteins: Disparate Origins but Convergent Parasitic Strategies. American Society for Microbiology 191, 6193-6202) Perking R.
Group II introns; Group II introns are both catalytic RNAs (ribozymes) and mobile retro elements that were discovered almost ~20 years ago. It has been suggested that eukaryotic mRNA introns might have originated from the group II introns present in the alpha-proteobacterial progenitor of the mitochondria. Bacterial group II introns are of considerable interest not only because of their evolutionary significance, but also because they could potentially be used as tools for genetic manipulation in biotechnology and for gene therapy.
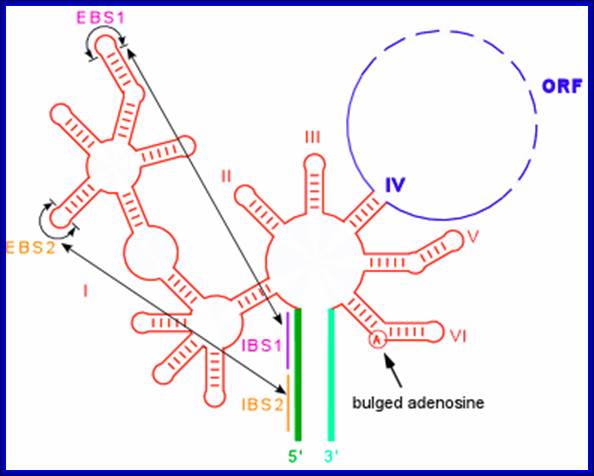
Group II introns are large catalytic RNAs (ribozymes) and mobile retro elements [reviewed by Pyle & Lambowitz (2006)] .
This figure summarizes what is known about the splicing mechanisms and mobility of bacterial group II introns, and describes the recent development of group II intron-based gene-targeting methods. Bacterial group II intron diversity, evolutionary relationships, and behavior in bacteria is very interesting. Group II introns are a class of mobile DNAs consisting of a catalytic RNA (ribozyme) and an intron- encoded protein IEP.
A list of processing enzymes found in cells:
�����������������������������������������������
|
Name of the Enzymes |
Name of the Gene |
Substrate |
Type of activity |
|
RNase-P |
rrPA, B |
5� of tRNA |
Endo |
|
RNase-BN |
? |
3� of tRNA |
Exo |
|
RNase-D |
rnD |
3� of tRNA |
Exo |
|
RNase-T |
? |
3� of tRNA CCA |
Exo |
|
RNase-III |
PacIp Rntp Ip |
rRNA, snRNAs, snoRNAs and mRNA |
Endo |
|
RNase-R |
? |
rRNA and mRNA |
Exo |
|
RNase-E |
RnE |
5s RNA, tRNA |
Endo |
|
RNase-II |
Rnb |
3� of unstructured RNA |
exo |
|
Polynucleotide phosphorylase |
Pnp |
Unstructured RNA |
Exo,cleaves mRNAs |
|
RNase-H |
Rnn-A,B RNA:DNA hybrid |
Cleaves only RNA part of cDNA |
Endo, sometimes exo also |
|
RNase-F |
|
rRNA-tRNA hybrid 3�end |
Endo |
|
M16 |
|
5� and 3� end of pre23s rRNA |
Endo |
|
M23 |
|
Pre 23srRNA |
Endo |
|
M5 |
|
5, and 3� of 5s rRNA |
Endo |
|
RNAse MRP |
|
5srRNA |
|
|
|
|
|
|
|
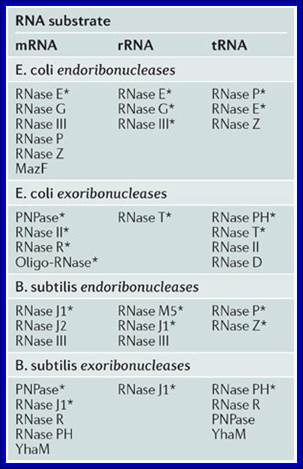
E.coli RNases: |
|
|
|
|
Endonucleases: RNaseE, ,III, P, Z and MazF |
|
Act on mRNA |
|
|
RNaseE, C, III |
|
Act on rRNAs |
|
|
RNaseP, E Z |
|
Act on tRNAs |
|
|
Exonucleases: |
|
|
|
|
PNPase, RNaseH, R oligoRNase |
|
Act on mRNAs |
|
|
RNaseT |
|
Act on rRNA |
|
|
|
|
|
|
|
RNase PH, T,II,O |
|
act on tRNAs |
|
|
|
|
|
|
�����������
E. coli enzymes;
Detection of 5′- and 3′-UTR-derived small RNAs and cis-encoded antisense RNAs in Escherichia coli; Mitsuoki Kawano1et al.
Evidence is accumulating that small, noncoding RNAs are important regulatory molecules. Computational and experimental searches have led to the identification of ∼60 small RNA genes in Escherichia coli. However, most of these studies focused on the intergenic regions and assumed that small RNAs were >50 ntds long. Thus, the previous screens missed small RNAs encoded on the antisense strand of protein-coding genes and small RNAs of <50 nt. To identify additional small RNAs, we (the above authors) carried out a cloning-based screen focused on RNAs of 30�65 nt. In this screen, we identified RNA species corresponding to fragments of rRNAs, tRNAs and known small RNAs. Several of the small RNAs also corresponded to 5′- and 3′-untranslated regions (UTRs) and internal fragments of mRNAs. Four of the 3′-UTR-derived RNAs were highly abundant and two showed expression patterns that differed from the corresponding mRNAs, suggesting independent functions for the 3′-UTR-derived small RNAs. We also detected three previously unidentified RNAs encoded in intergenic regions and RNAs from the long direct repeat and hok/sok elements. In addition, we identified a few small RNAs that are expressed opposite protein-coding genes and could base pair with 5′ or 3′ ends of the mRNAs with perfect complementarity.