RESPIRATION
Biological Oxidation:
All living organisms, without any exception require free chemical energy for their multitudes of physiological functions.� In most of the cases this energy is supplied in the form ofATP molecules.� However, in many cases, GTP, CTP, phosphocreatine, NADH + H*, Arginine phosphate, PEP etc., also supply the energy for specific reactions.
Mitochondrial cristae.
The ATP molecule is made up of Adenosine and three phosphates.� Three phosphates are linked by high energy or anhydride bonds.� These high energy bonds are called swigle bonds.� Break down of these terminal bonds by specific enzymes yield 7.2 K. Cals/mol �1 of free energy.� This energy can be utilized for various functions like transmission of nerve stimulus, muscular contraction, osmoregulation, maintenance of body temperature, transpiration of materials etc.� At the same time the energy present in these terminal bonds can be transferred to other molecules to build up macromolecules like carbohydrates, proteins, nucleic acids, fats and other reserve food substances.
�
Mitochondrial Cristae; mitochondria surrounded by ER
Plants and animals use various stored food materials as their energy source: starch, glycogen (it is animal starch), fats and proteins act as the energy sources.� Organisms, during the course of evolution, probably 2 or 2.2 billion years ago, with the onset of O2 liberation by photosynthetic organisms, have evolved a unique mechanism, by means of which various food sources are systematically oxidized in a controlled degradative process, in which the energy that is released is stored in the high energy chemical bonds of ATPs and other molecules.� Thus, they are made available for cellular metabolism.� This process is often termed as �Biological Oxidation�.
Biological oxidation per-se involves stepwise oxidation of reserve food materials in a controlled but stepwise process. Depending upon the utilization of oxygen or without oxygen- oxidation of food substances this process can be classified into anaerobic and Aerobic oxidation.� Most of the organisms perform Aerobic oxidation, but some bacteria, yeast and even some higher animals exhibit Anaerobic oxidation, of which aerobic process is more complicated and yields energy 8-10 times the anaerobic process.
Biological Oxidation Process-Aerobic:
Irrespective of plants and animals, aerobic process requires mitochondria as energy producing organelles.� These structures are found in almost all organisms except, blue green algae, bacteria, Mycoplasma and viruses.� The number, position, shape and size of these structures vary from organisms to organisms, and from organ to organ and the metabolic status has a significant in the mitochondrial activity.

utilization of a carbohydrate-Glycolysis.
Biochemical Pathway of aerobic respiration:
Reserve food materials like Carbohydrates, Fats and Proteins are first subjected to hydrolysis by specific enzymes.� As a result, simpler compounds like Glucose, Keto acids and Acetyl Co-A are released into the Cytoplasm.� From this pool various components are drawn into the main oxidative pathway.� For example, carbohydrates, like starch or sugars are degraded into their respective Glucose units by either starch phosphorylase or Amylases.� Similarly, proteins are degraded by various proteinases into amino acids.� Fats are subjected to B-Oxidation to produce Acetyl-Co. A molecule.
The entire process of biological oxidation takes places in 3 steps, at 3 different sites.� The first step is Glycolysis.� It takes place in cytoplasm.� It does not require oxidation for its function. The glycolytic products are drawn into mitochondrial matrix in which Krebs cycle operates with the utilization of glycolytic products.� The final or the third step occurs in /on the inner mitochondrial membranes.� Some of Krebs cycle products are used up by the membranes and they are subjected to a process called terminal oxidation or oxidative phosphorylation.� This step requires oxygen for its function.
Glycolysis an anaerobic pathway (EMP Pathway):
Glucose molecules are directly utilized in the first step of this pathway.� There are many biochemical steps and require specific enzymes.� In the first phase of this process 1 or 2 molecules of ATP are consumed as priming reactions.� On the other hand, in the latter part of these reactions energy rich products are produced.� Glycolysis takes place exclusively in cytoplasm.
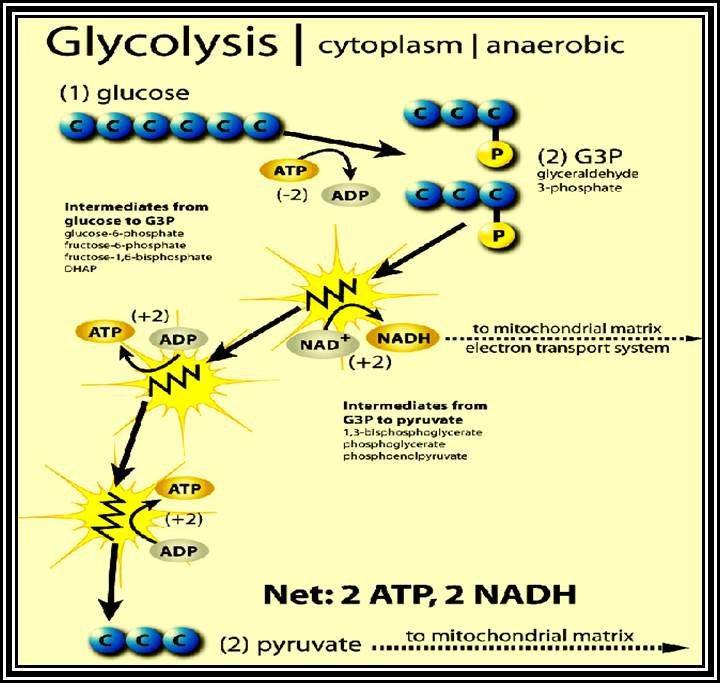
Glycolytic steps.
Glucose is primed by ATP with the help of an enzyme-Hexokinase.� If the glucose is already phosphorylated in the first position, as in the case of starch-phosphorylation, Glucose-1, P is converted to Glucose-6-P by phosphoglucomutase.
Glucose is isomerized to Fructose-6-P by an enzyme Phosphoglucomutase-isomerase.
������ 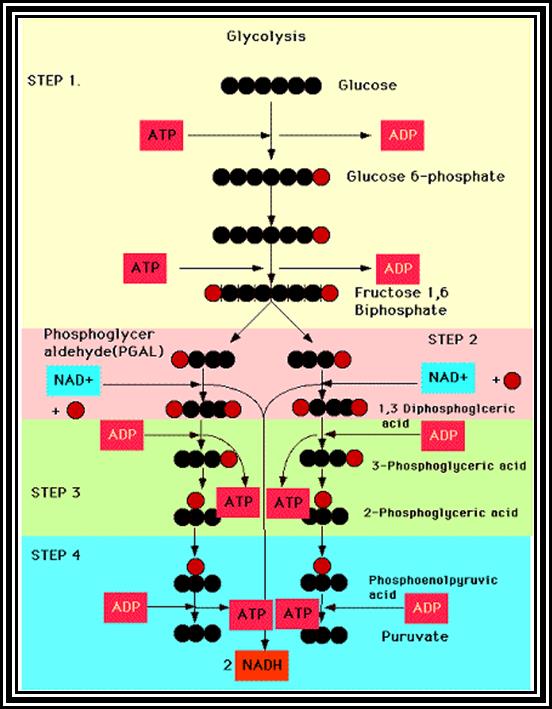
Overall view of Glycolysis.
Fructose �6P is further energized by another molecule of ATP by phosphofructo kinase.
Highly energized 6 carbon Fructose diphosphate is acted upon by aldolase and it is split into 3 carbon compounds like phospho-glyceraldehde and Dihydroxyacetone�P.� These products are interconvertible by Triose isomerase.
Phosphoglyceraldehyde is oxidized to 1, 3 � diphosphoglyceric acid by Triose phosphate dehydrogenase.� One molecule of NADH + H* is produced.
Energy rich 1, 3 � diphospho-glycerate is converted to 3-Diphosphoglycerate by phosphoglyceryl kinase.� A molecule of ATP is synthesized by substrate phosphorylation.
Phosphate in 3rd position is shifted to 2nd position by phospho glycero-mutase.
A molecule of water is removed from 2-PGA by Enolase.
Energy rich 2-PEP donates energy rich phosphate bond to ADP to form ATP, by pyruvate kinase.
This is another event of substrate phosphorylation.
In the above glycolytic process, the two steps are of ATP energy utilizing reactions.� There are three reactions in which high energy bonds are transferred and conserved in NADH + H and ATP molecules.� In this process one molecule of 6C carbon glucose yields 2 molecules of 3 Carbon compounds like PGAlD and DiHAP which are interconvertible, but PGALD is subjected to dehydrogenation reactions.� Thus, whatever happens of PGALD also happens to DiHAP through the conversion of it into PGALD.� Furthermore, one PGALD at the end of glycolysis yields 1 NADH + H and 2 ATP molecules.� So, one molecule of glucose on complete glycolysis yields 2 NADH + H, 4 ATP and 2 Pyruvate molecules.� This process is common for both aerobic and anaerobic respiration.
Alcoholic Fermentation or Partial Biological Oxidation: Anaerobic respiration:
Certain organisms like yeasts (under anaerobic conditions), bacteria like Clostridium (an obligate anaerobic bacterium), Lactic acid bacteria and muscular respiration in higher animals are some of the examples where cells respire anaerobically.� This process is a partial biological oxidation in which a part of the glucose is oxidized intra-molecularly and 2 molecules of CO2 are released.� And the ultimate products are ethanol or lactate.� This is named after the discoverer of this process as EMP pathway.� (Embden, Mayer-off, Parness�s pathway).� This process has been commercially exploited very well in brewing and chemical industries.
In this pathway, glucose is subjected to series of oxidative processes and 2 molecules of pyruvates are formed. �The entire process is similar to glycolytic pathway.� But pyruvate produced at the end of glycolysis is either converted to ethanol (in yeasts) or to lactate (in the case of lactate bacteria or Muscular respiration).
Alcoholic Fermentation:� Yeast cells are capable of oxidizing glucose and such substrates either in the presence of oxygen or absence of oxygen. Under anaerobic conditions yeasts use glucose and convert it into pyruvate by glycolytic reactions.� However, Pyruvate thus produced is subjected to decarboxylation first and then reduction.
�
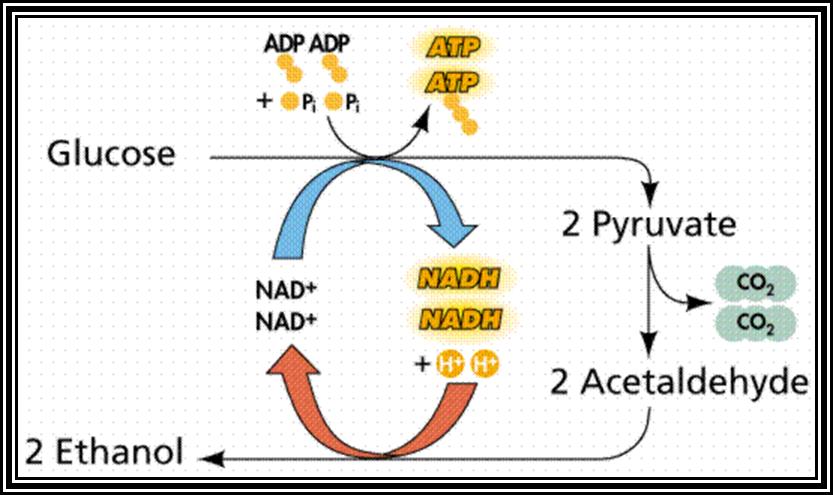
��������������������� Anaerobic process
In this process, only two carbon atoms of 6 carbon glucose are released by intra-molecular oxidation to 2 CO2.� Two molecules of NADH + H,* produced in glycolytic process, are used up in the reduction of acetaldehyde to Ethanol.� Thus, the net gain is just 2 ATPs per Glucose molecule.
Lactate Formation:� Lactate formation by anaerobic process is found to operate, in not only lactate bacteria (responsible for curdling of milk) but also in muscular respiration.� As in the case of alcoholic fermentation, pyruvate generated by glycolytic process is directly reduced by lactate dehydrogenase in which 2 NADH + H* molecules produced during glycolytic steps are used up.� Again, the net gain is just 2 molecules of ATP / glucose.� It is interesting to note that though human beings are highly evolved, they have retained evolutionarily primitive biochemical process like anaerobic fermentation.� This process is more predominant, when human beings are subjected to violent exercises. The fatigue, the man experiences during such activities is due to accumulation of Lactate in muscles which may result in cramps.
This is due to the deficit supply of oxygen.� However, man recovers, when he regains sufficient supply of oxygen by utilizing the stored lactate.
Krebs Cycle: A Mitochondrial Process:
A molecule of glucose on glycolysis yields two molecules of 3 carbon compounds called pyruvate.� Pyruvates thus synthesized in cytoplasm are transported across the membranes into mitochondrial matrix.� During this process pyruvate is subjected to a series of complex reactions.
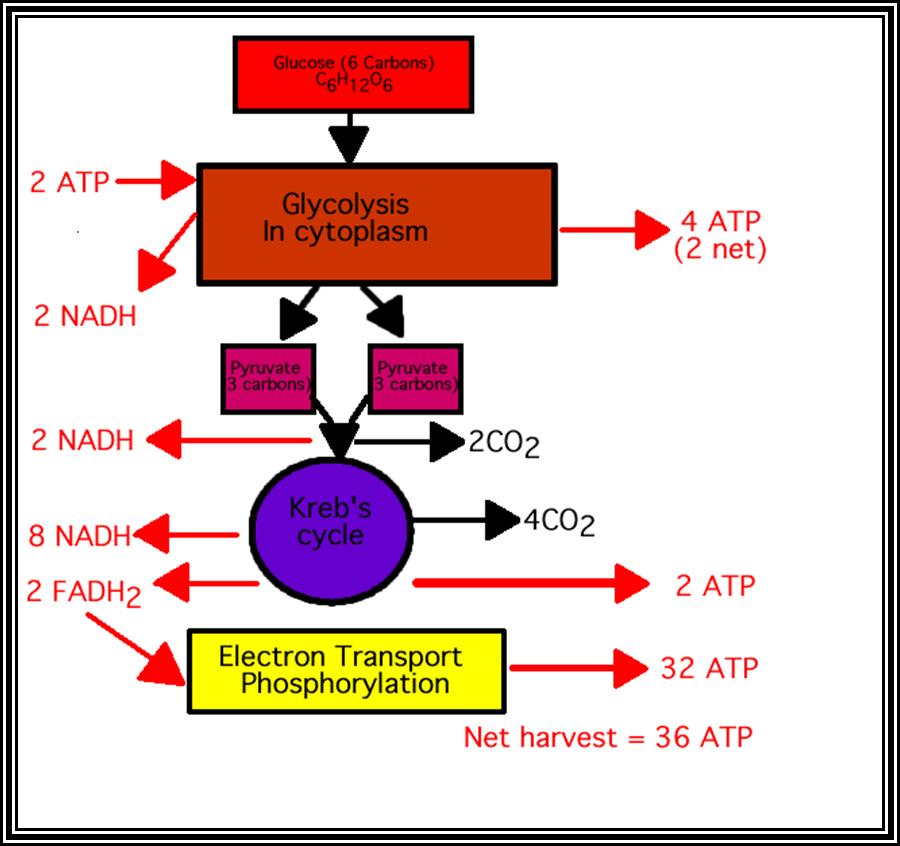
Glycolysis in Cytoplasm. Then Kreb�s cycle in Mitochondrial matrix
����������������������������������� Glycolysis
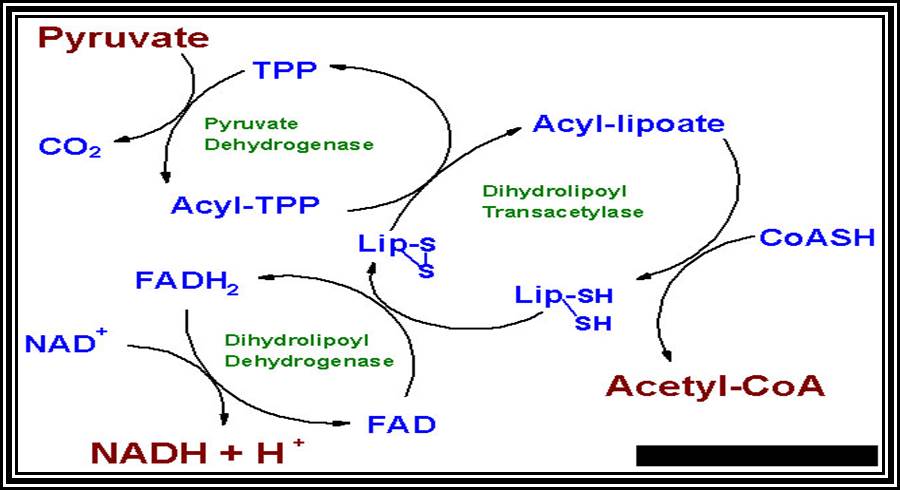
In these reactions decarboxylation and dehydrogenation reactions take place and Acetyl-Co-A is liberated into the matrix.� The enzyme that is responsible is a multiple enzyme complex called pyruvate dehydrogenase.
Acetyl Co-A, as it is transported into mitochondrial fluid, it is drawn by a group of enzymes into Krebs cycle (it is named after the discoverer of this cycle i.e., Hans Krebs).
The 2 carbon Acetyl Co. A combine with a Ketonic 4 carbon oxaloacetate and undergoes a condensation process by an enzyme called citrate synthetase to form 6 carbon citrates.
Citrate in turn undergoes a successive dehydration and hydration steps to form Isocitrate.� The enzyme responsible for this process is Aconitase.
Then Isocitrate is subjected to a sequential dehydrogenation and decarboxylation reactions by Isocitrate dehydrogenase (the Co-enzyme is NAD or NADP).
� Alpha-Ketoglurate is further decarboxylated and dehydrogenated to Succinate by L-Ketoglutarate dehydrogenase.� In this, a high energy bond is transferred to GDP + Pi to form GTP which is another case of substrate phosphorylation.
Succinate is again converted to Fumerate by a Flavoprotein enzyme called Succinate dehydrogenase where the Co-enzyme is FAD.
Fumerate is then converted to Malate with addition of one molecule of water.� The enzyme responsible is Fumerase.
Malate is subjected to Malate dehydrogenase reaction to form oxaloacetate.
The Oxaloacetate thus produced again combines with Acetyl-Co.A to form citrate and Krebs cycle continues.
Krebs cycle which is also called Tricarboxylic acid (TCA) cycle is very important for, it provides not only energy rich compound like 4 NADH + H*, 1 FADH2 and 1 GTP but also it provides some important intermediate compounds for other metabolic pathways.
In total, 2 pyruvates yield 8 molecules of NADH + H* 2 FADH2 and 2 GTP (2 GTP are converted to 2 ATP) and 6 mol. of CO2.
When glycolysis and Kerbs cycle are summed up, 1 molecule of glucose yields 10 NADH + H, 2 FADH2, 6 ATP and 6 CO2. It is important to note that all 6 carbons of glucose molecule are oxidized, and so it can be considered as complete oxidations.
Terminal Oxidation
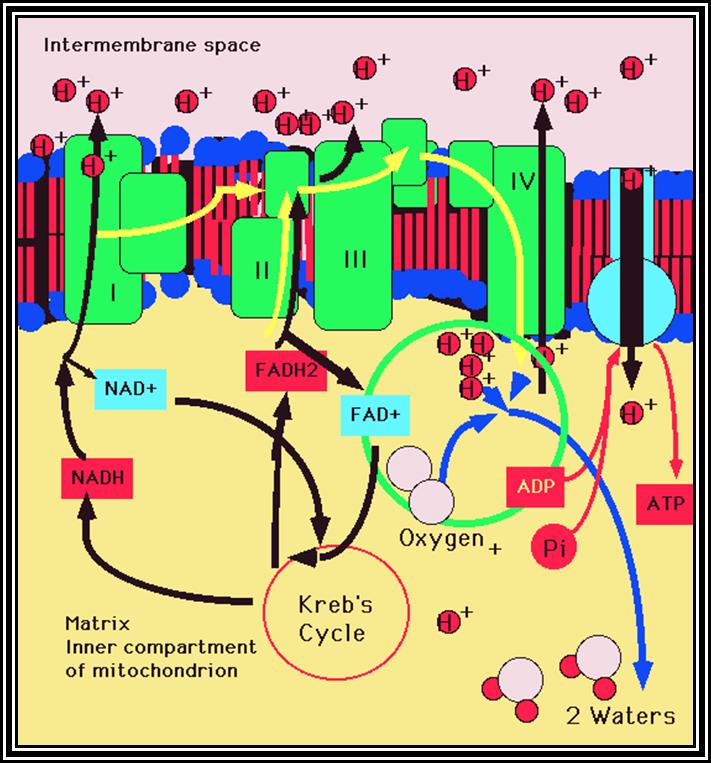
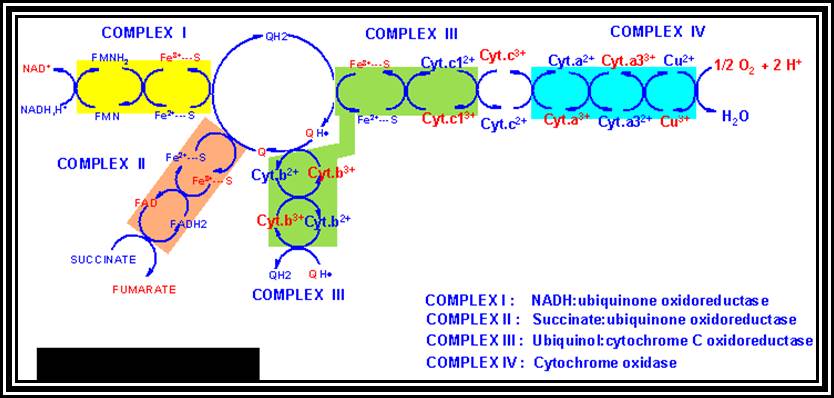
A Phenomenon of Oxidative phosphorylation:�Reduced products like NADH + H and FADH2 that are synthesized during glycolytic and Krebs cycle reactions are energy rich molecules. In order to trap and transfer this bond energy to ATP molecules, the reduced products are taken up by the enzymes found in the inner membrane and the same are subjected to a sequential Red-Ox process.� The enzymes that are involved in this process are Fe-S.FMN bond and Fe-S-FAD bond reductase, NADP reductase Co enzyme Q (a labile molecule), Cyt. B. Oxidase, Cyt C and Cyt a/a3 oxidase (copper containing enzyme) and these arranged vectorially and sequentially.
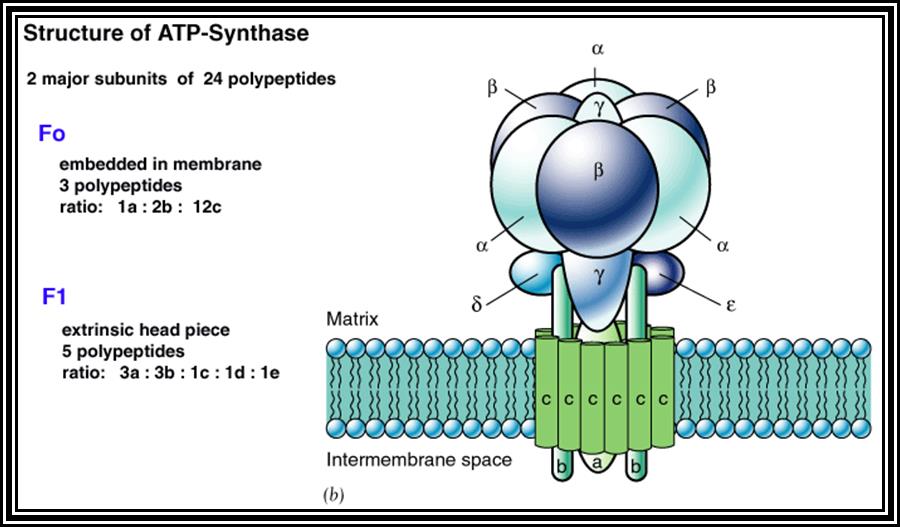
Of these, Fe-FMN accepts hydrogen from NADH + H, Fe- S-FAD accepts hydrogen from FADH2 and Co-Q accepts hydrogen from both FMNH2 and FADH2.� However, the Cytochrome containing Fe accepts only electrons and H+ are released into the inner membrane.� When electrons reach the terminal enzyme Cyt a3, they are transferred to � O2; simultaneously 2H+ are also accepted by �O2 to form a molecule of water.� During this process of Terminal Oxidation, simultaneous oxidation � reduction reactions occur.� Depending upon the red-ox potential of individual components, energy is released at 3 different sites. This energy is used up by ATP synthetase enzyme to synthesize ATP from ADP + Pi.� Hence, this phenomenon is also called oxidative phosphorylation.
The most fascinating aspect of terminal oxidation is the utilization of energy that is released at 3 sites during the passage of electrons through the respiratory chain. Though the mechanism of energy coupling had remained unsolved, many theories have been proposed from time to time.� Slater and Chance, proposed chemical coupling theory in 1939 and envisaged that during electron transport, the energy that is released at the said sites are taken up by an unknown intermediate compound with high energy bond, then this bond is transferred to ADP and Pi to form ATP.� So far no one is able to detect or identify such high energy intermediates.� But Peter Mitchell (1961) came out with an interesting hypothesis called Chemiosmotic hypothesis.� He visualized, that the components of electron transport chain are vectorially arranged (different components at different sides of the membrane) various components accept NADH + H and FADH2 at different sites from inner surface and extrude proteins H+ proton on the outer surface but the electrons are passed through the electron transport chain. This extrusion of protons to outside, builds up a proton gradient or proton motive force, which is enough to bring about the formation of high energy phosphate bond between ADP + Pi to form ATP.� This theory became very popular and soon it was widely accepted.� Peter Michell was awarded a Nobel Prize in chemistry in 1978.
However, the opponents of Mitchell theory started getting new facts which were contrary to the observation of Peter Mitchell�s supporters.� Boyer in 1974 proposed an alternative theory called conformational coupling theory.
�
Terminal Oxidation involving organized Cytochromes a, b and c.
����������������������������������� Terminal Oxidation
This was based on the conformational changes observed during mitochondrial ATP synthesis.� Certain Actin like components found in the inner membrane of mitochondria to play a significant role in the conformation coupling process.� Currently this theory is gaining more support.
����������������������������������������������������������� ATP synthase complex.
Factors
As respiration involves very many biochemical steps, many factors like water, substrate, oxygen, CO2 temperature etc., control the rate of respiration.
1. Water:� Water being the medium in which all the cellular components are either suspended or dissolved, the rate of reaction depends upon the concentration of the water in a given system.� If a dry seed is taken for measuring its rate of reactions depend upon the concentration of the water in a given system.� If a dry seed is taken for measuring its rate of respiration, it is seen that the respiration is at its minimum, this is because the amount of water found in dry or resting seeds is just 2-8%.� Hence movement of molecules is restricted to a small space, so collisions between the reactants and enzymes become infrequent and the rate of metabolism will be very low.� As the dry seeds are allowed to imbibe water, its concentration within the cells increase and rate of metabolism, consequently the rate of respiration increases significantly.
2. Substrate:��Respiration or biological oxidation virtually depends upon its substrates for the release of energy.� Most of the plants / animals� store starch/ glycogen as the food material.� Still there are many organisms, which along with carbohydrates, store oil and proteins (E.g., Castor bean, ground nut seed, cereal grain etc).� When conditions are favorable for respiration, plants utilize carbohydrates first, and then they utilize fats and proteins in succession.� Whether plants are utilizing starch, fats or proteins as the substrate for respiration, it can be determined by measuring Respiratory Quotient or RQ. Respiratory quotient denotes the ratio between the CO2 released to the oxygen consumed.
��� ������� ����� CO2 released
������� RQ� = -------------------
���������� ����� Q2 consumed
Different food sources exhibit different R.Q. E.g. R.Q. of starch or carbohydrate is 1; R.Q. of fats or oil is = 0.7, R.Q. of proteins ranges from 0.7 � 0.9 and organic acid shows 1.2.4.
The rate of respiration further depends upon the availability of substrates. With the increase in the substrate concentration the rate of respiration also increases; but beyond certain level the rate remains constant because respiration depends upon the concentration of enzymes which generally remain constant in a given set of conditions.
3, Temperature:� Heat is another form of energy and it provides momentum for the molecules to move.� Increase in temperature increases the energy of the system and the rate of movement of molecules also increases.� This increase in the rate of movement of molecules increases the rate of collision between the respiratory enzymes and the substrates.� Thus, the rate of respiratory enzymes and the respiration is expressed as the function of temperature; one finds the rate is minimum at lower temperature.� With the increase of every 100C the rate doubles and at a particular range of temperature the rate is maximum.� This range of temperature is called the optimum temperature.� This optimum varies from plant to plant for it is genetically determined.� But higher temperature is always fatal because, enzymes which are functional molecules, breakdown and become nonfunctional.
4. Oxygen:� Aerobes require oxygen for the oxidation of substrates.� The majority of plants / animals are obligate aerobes.� However, plants like yeasts can exist as aerobes as well as anaerobes and their adaptability to these conditions is remarkable.� But these are some bacteria which are obligate anaerobes and the presence of oxygen is fatal for these organisms.
In aerobes, oxygen acts as one of the substrates, because oxygen ultimately receives electrons and protons at the terminal end of electron transport chain.� And this is extremely important.� Any inhibition of this step inhibits the production of ATP and it is fatal.
In plants, respiration takes place both at day time as well as at night time.� But the respiration is very low in leaves at day times particularly in tissues where cells are engaged in photosynthesis.� This is because photosynthesis provides all the energy that is required for the cellular metabolism.
Generally, the increase in O2 concentration enhances the rate of respiration but beyond certain level the rate remains constant.� In facultative anaerobes like yeasts, when they are respiring anaerobically, if oxygen is supplied, alcoholic fermentation is completely stopped and the entire process now switches over to aerobic metabolism.� This effect is called Pasteur�s effect.� During anaerobic process, the number of mitochondria per yeast cell is as low as one or two.� When only oxygen is provided to such anaerobic cells, there is a sudden spurt in the multiplication of mitochondria and in about 30 minutes or so the number of mitochondria and in about 30 minutes or so the number of mitochondria increases to 100-150 per cell.� If such cells, which are fully geared up for aerobic respiration are subjected to anaerobic conditions, one sees a dramatic change of sequence of events, where mitochondria undergo breakdown and their number gets reduced to 1-2 / cell.
5. CO2:� �The oxidation of substrates during respiration yields CO2 as one of its products.� Normally CO2 diffuses out through transpiration system.� However, if the concentration of CO2 builds up to a greater extent CO2 acts as an inhibitor of respiration and it may have a fatal effect on living system.� Generally, plants do not suffer from the excess of CO2.� On the other hand, increased CO2 favors photosynthesis and the yield increases.
6. �Inhibitors:��Many drugs like DNP, Rotenone, Antimycin, Cyanide etc., inhibit respiratory oxidation.� This is because these drugs have specific inhibitory effect on specific respiratory enzymes.� For example, cyanide binds to cytochrome oxidase and inhibits the transport of electrons to O2 and stops oxidation which results in the complete inhibition of ATP production.� That is how cyanide causes death.� But some plants like Aeroids are insensitive to cyanide poisoning, for they have a different mechanism for their electron transport chain.
7. Internal Factors:� The cellular factors like enzyme synthesis, the availability of CO enzymes, and cofactors and other nutrients are very essential for the normal metabolic processes.� If any of these factors is either deficient or absent, it prevents the enzymes from doing their normal function and this causes the inhibition of respiration.�